Targeted next-generation sequencing - a promising approach in the diagnosis of Mycobacterium tuberculosis and drug resistance
- PMID: 39419957
- PMCID: PMC12137396
- DOI: 10.1007/s15010-024-02411-w
Targeted next-generation sequencing - a promising approach in the diagnosis of Mycobacterium tuberculosis and drug resistance
Abstract
Targeted next-generation sequencing (tNGS) offers a high-throughput, culture-independent approach that delivers a comprehensive resistance profile in a significantly shorter turn-around time, making it promising in enhancing tuberculosis (TB) diagnosis and informing treatment decisions. This study aims to evaluate the performance of tNGS in the TB diagnosis and drug resistance detection of Mycobacterium tuberculosis (MTB) using MTB clinical isolates and bronchoalveolar lavage fluid (BALF) samples. A total of 143 MTB clinical isolates were assessed, tNGS, phenotypic antimicrobial susceptibility testing (AST), and AST based on whole genome sequencing (WGS) exhibited high concordance rates, averaging 95.10% and 97.05%. Among 158 BALF samples, culture, Xpert MTB/RIF, and tNGS reported 29, 70 and 111 positives, respectively. In the confirmed cases with etiological evidence (smears, cultures, or molecular test), the positive rate of tNGS (73/83, 87.95%) was higher than that of Xpert MTB (67/83, 80.72%). Additionally, 45% (27/60) of clinically diagnosed cases (with imaging or immunological evidence) were positive for tNGS. Further validation on the discrepant results between tNGS and Xpert MTB/RIF with droplet digital PCR (ddPCR) yielded 35 positives, tNGS detected all, and Xpert MTB/RIF only identified 6 positives. In conclusion, tNGS demonstrates robust and rapid performance in the identification of MTB and its associated drug resistance, and can be directly applied to clinical samples, positioning it as a promising approach for laboratory testing of tuberculosis.
Keywords: Mycobacterium tuberculosis; Diagnosis; Drug resistance; Targeted next-generation sequencing.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethical approval: This study was approved by the Ethical Committee of Shanghai Pulmonary Hospital. Conflict of interest: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
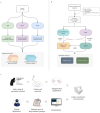
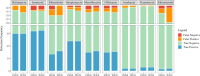
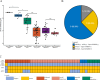
References
-
- WHO. Global tuberculosis report 2023. 2023.
-
- WHO. The use of next-generation sequencing for the surveillance of drug-resistant tuberculosis: an implementation manual. 2023.
-
- WHO. Use of targeted next-generation sequencing to detect drug-resistant tuberculosis: rapid communication, July 2023. 2023.
MeSH terms
Substances
Grants and funding
- 20224Y0129/Shanghai Municipal Health Commission's Special Youth Project for Clinical Research in the Health Industry
- . GWVI-11.2-YQ52/Shanghai Three-year (2023-2025) Action Plan to Strengthen the Public Health System
- GWVI-11.1-05/Shanghai Three-year (2023-2025) Action Plan to Strengthen the Public Health System
- 22Y11902600/Shanghai Science and Technology Innovation Action Plan, Medical Innovation Research Special Project
LinkOut - more resources
Full Text Sources
Medical

