Multiple beta cell-independent mechanisms drive hypoglycemia in Timothy syndrome
- PMID: 39420001
- PMCID: PMC11487186
- DOI: 10.1038/s41467-024-52885-3
Multiple beta cell-independent mechanisms drive hypoglycemia in Timothy syndrome
Abstract
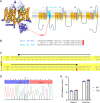
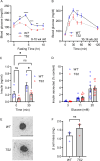
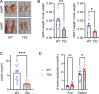
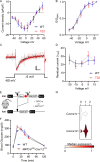
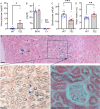
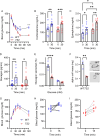
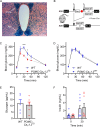
The canonical G406R mutation that increases Ca2+ influx through the CACNA1C-encoded CaV1.2 Ca2+ channel underlies the multisystem disorder Timothy syndrome (TS), characterized by life-threatening arrhythmias. Severe episodic hypoglycemia is among the poorly characterized non-cardiac TS pathologies. While hypothesized from increased Ca2+ influx in pancreatic beta cells and consequent hyperinsulinism, this hypoglycemia mechanism is undemonstrated because of limited clinical data and lack of animal models. We generated a CaV1.2 G406R knockin mouse model that recapitulates key TS features, including hypoglycemia. Unexpectedly, these mice do not show hyperactive beta cells or hyperinsulinism in the setting of normal intrinsic beta cell function, suggesting dysregulated glucose homeostasis. Patient data confirm the absence of hyperinsulinism. We discover multiple alternative contributors, including perturbed counterregulatory hormone responses with defects in glucagon secretion and abnormal hypothalamic control of glucose homeostasis. These data provide new insights into contributions of CaV1.2 channels and reveal integrated consequences of the mutant channels driving life-threatening events in TS.
© 2024. The Author(s).
Conflict of interest statement
G.S.P. is on the scientific advisory board of Tevard Biosciences. All other Authors declare no competing interests.
Figures
References
-
- Splawski, I. et al. Ca(V)1.2 calcium channel dysfunction causes a multisystem disorder including arrhythmia and autism. Cell119, 19–31 (2004). - PubMed
-
- Yates, D., Yates, A. & Collyer, T. A life-threatening complication of the arterial tourniquet in Timothy syndrome. Paediatr. Anaesth.17, 492–495 (2007). - PubMed
-
- Joseph-Reynolds, A., Auden, S. & Sobczyzk, W. Perioperative considerations in a newly described subtype of congenital long QT syndrome. Paediatr. Anaesth.7, 237–241 (1997). - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

