Methionine-SAM metabolism-dependent ubiquinone synthesis is crucial for ROS accumulation in ferroptosis induction
- PMID: 39420002
- PMCID: PMC11487270
- DOI: 10.1038/s41467-024-53380-5
Methionine-SAM metabolism-dependent ubiquinone synthesis is crucial for ROS accumulation in ferroptosis induction
Abstract
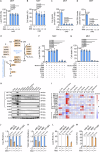
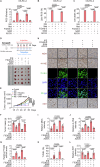
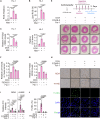
Ferroptosis is a cell death modality in which iron-dependent lipid peroxides accumulate on cell membranes. Cysteine, a limiting substrate for the glutathione system that neutralizes lipid peroxidation and prevents ferroptosis, can be converted by cystine reduction or synthesized from methionine. However, accumulating evidence shows methionine-based cysteine synthesis fails to effectively rescue intracellular cysteine levels upon cystine deprivation and is unable to inhibit ferroptosis. Here, we report that methionine-based cysteine synthesis is tissue-specific. Unexpectedly, we find that rather than inhibiting ferroptosis, methionine in fact plays an essential role during cystine deprivation-induced ferroptosis. Methionine-derived S-adenosylmethionine (SAM) contributes to methylation-dependent ubiquinone synthesis, which leads to lipid peroxides accumulation and subsequent ferroptosis. Moreover, SAM supplementation synergizes with Imidazole Ketone Erastin in a tumor growth suppression mouse model. Inhibiting the enzyme that converts methionine to SAM protects heart tissue from Doxorubicin-induced and ferroptosis-driven cardiomyopathy. This study broadens our understanding about the intersection of amino acid metabolism and ferroptosis regulation, providing insight into the underlying mechanisms and suggesting the methionine-SAM axis is a promising therapeutic strategy to treat ferroptosis-related diseases.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
- 32101028/National Natural Science Foundation of China (National Science Foundation of China)
- 2020T130090/China Postdoctoral Science Foundation
- OT2 OD030544/OD/NIH HHS/United States
- 2019M661190/China Postdoctoral Science Foundation
- 32101048/National Natural Science Foundation of China (National Science Foundation of China)
LinkOut - more resources
Full Text Sources

