OTUD5 promotes end-joining of deprotected telomeres by promoting ATM-dependent phosphorylation of KAP1S824
- PMID: 39420004
- PMCID: PMC11486905
- DOI: 10.1038/s41467-024-53404-0
OTUD5 promotes end-joining of deprotected telomeres by promoting ATM-dependent phosphorylation of KAP1S824
Abstract
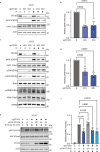
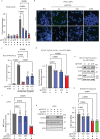
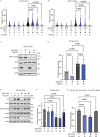
Appropriate repair of damaged DNA and the suppression of DNA damage responses at telomeres are essential to preserve genome stability. DNA damage response (DDR) signaling consists of cascades of kinase-driven phosphorylation events, fine-tuned by proteolytic and regulatory ubiquitination. It is not fully understood how crosstalk between these two major classes of post-translational modifications impact DNA repair at deprotected telomeres. Hence, we performed a functional genetic screen to search for ubiquitin system factors that promote KAP1S824 phosphorylation, a robust DDR marker at deprotected telomeres. We identified that the OTU family deubiquitinase (DUB) OTUD5 promotes KAP1S824 phosphorylation by facilitating ATM activation, through stabilization of the ubiquitin ligase UBR5 that is required for DNA damage-induced ATM activity. Loss of OTUD5 impairs KAP1S824 phosphorylation, which suppresses end-joining mediated DNA repair at deprotected telomeres and at DNA breaks in heterochromatin. Moreover, we identified an unexpected role for the heterochromatin factor KAP1 in suppressing DNA repair at telomeres. Altogether our work reveals an important role for OTUD5 and KAP1 in relaying DDR-dependent kinase signaling to the control of DNA repair at telomeres and heterochromatin.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
- 812829/EC | EU Framework Programme for Research and Innovation H2020 | H2020 Priority Excellent Science | H2020 Marie Skłodowska-Curie Actions (H2020 Excellent Science - Marie Skłodowska-Curie Actions)
- 2019-2/12826/KWF Kankerbestrijding (Dutch Cancer Society)
- institutional grant to the Netherlands Cancer Institute/KWF Kankerbestrijding (Dutch Cancer Society)
- Institutional grant to the Netherlands Cancer Institute/Dutch Ministry of Health, Welfare and Sport | Rijksinstituut voor Volksgezondheid en Milieu (Netherlands National Institute for Public Health and the Environment)
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

