Longitudinal liquid biopsy predicts clinical benefit from immunotherapy in advanced non-small cell lung cancer
- PMID: 39420036
- PMCID: PMC11486993
- DOI: 10.1038/s41698-024-00704-9
Longitudinal liquid biopsy predicts clinical benefit from immunotherapy in advanced non-small cell lung cancer
Abstract
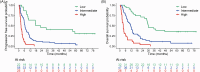
High heterogeneity in clinical benefit characterizes the use of immune checkpoint inhibitors (ICIs) in non-small cell lung cancer (NSCLC). We prospectively enrolled 113 advanced NSCLC patients treated with ICIs and performed liquid biopsy at the time of ICI start (T1), after 3 weeks (T2) and at the time of radiological evaluation (T3). Molecular variables were associated with outcome endpoints: cfDNA quantification, its dynamic change (∆T2-T1), variant allele frequency (VAF) of the gene with the highest frequency detected at baseline with NGS (maxVAF) and its dynamic change (∆T2-T1). At multivariate analysis, PD-L1 negativity, T1 cfDNA, cfDNA increase (∆T2-T1), and T2 VAF were significantly associated with shorter progression-free survival (PFS); PD-L1 negativity, squamous histology, T1 cfDNA, cfDNA (∆T2-T1) increase, and T2 maxVAF affected overall survival (OS). Among high PD-L1 expressing patients treated in first-line, elevated T2 maxVAF and cfDNA increase (∆T2-T1) correlated with worse PFS; higher T2 maxVAF and cfDNA increase (∆T2-T1) with worse OS. Derived integrated models were used to build nomograms and categorize different risk groups.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Hendriks, L. E. et al. Non-oncogene-addicted metastatic non-small-cell lung cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann. Oncol.34, 358–376 (2023). - PubMed
-
- Hendriks, L. E. et al. Oncogene-addicted metastatic non-small-cell lung cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up☆. Ann. Oncol.34, 339–357 (2023). - PubMed
-
- Gandhi, L. et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N. Engl. J. Med.378, 2078–2092 (2018). - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

