Phase 2 trial of PSMA PET CT versus planar bone scan and CT in prostate cancer patients progressing while on androgen deprivation therapy
- PMID: 39420060
- PMCID: PMC11487247
- DOI: 10.1038/s41598-024-75589-6
Phase 2 trial of PSMA PET CT versus planar bone scan and CT in prostate cancer patients progressing while on androgen deprivation therapy
Abstract
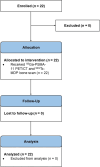
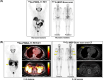
For prostate cancer patients who experience biochemical progression during androgen deprivation therapy (ADT), prostate-specific membrane antigen positron emission tomography/computed tomography (PSMA PET/CT) has not been prospectively compared to planar bone scan plus CT. This was a single-arm, head-to-head, prospective phase II trial (NCT04928820) designed to enroll 102 men with prostate cancer who experienced biochemical progression (rising prostate-specific antigen [PSA] ≥ 1 ng/mL) during ADT. All patients received 68Ga-PSMA-11 PET/CT and 99mTc-MDP planar bone scans. Each scan was interpreted by three central independent readers. The primary endpoint was the per-patient bone metastasis detection rate of PSMA PET/CT versus planar bone scan and CT. Secondary endpoints compared the number of bone metastases detected per patient and the inter-reader agreement of each imaging modality. Twenty-two men were enrolled between July 2021 and June 2022. Due to slow accrual following approval of PSMA PET radiotracers in the U.S. and a lack of a statistical signal between the two imaging modalities on interim analysis, this trial was closed early on October 2022. Median PSA was 8.5 ng/mL (interquartile range: 1.6-77.6). There was 100% agreement between the two scans. Six patients (27%) had negative findings and 16 patients (73%) had positive findings on both scans. PSMA PET/CT and bone scan plus CT detected an equal number of bone lesions for 14 patients (64%), PSMA PET/CT detected more bone lesions for six patients (27%), and bone scan plus CT detected more bone lesions for two patients (9.1%) (p = 0.092). The inter-reader agreement rates of PSMA PET/CT and bone scan plus CT were 96% and 82%, respectively (p = 0.25). In men with biochemical progression during ADT, 68Ga-PSMA-11 PET/CT and 99mTc-MDP planar bone scan plus CT had identical bone metastasis detection rates. Bone scan plus CT can continue to serve as a cost-effective and readily accessible restaging modality in patients with biochemical progression. ClinicalTrials.gov NCT04928820. Registered 16/06/2021.
Keywords: Androgen deprivation therapy; Biochemical recurrence; Bone metastases; Bone scan; Prostate-specific membrane antigen PET/CT.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- NCCN Clinical Practice Guidelines in Oncology: Prostate Cancer, Version 4. National Comprehensive Cancer Network. (2024). https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. Accessed on 21 Sept 2024. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

