Long term T cell response and safety of a tetravalent dengue vaccine in healthy children
- PMID: 39420169
- PMCID: PMC11487277
- DOI: 10.1038/s41541-024-00967-0
Long term T cell response and safety of a tetravalent dengue vaccine in healthy children
Abstract
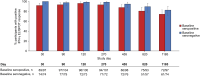
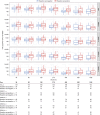
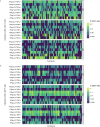
As robust cellular responses are important for protection against dengue, this phase 2 study evaluated the kinetics and phenotype of T cell responses induced by TAK-003, a live-attenuated tetravalent dengue vaccine, in 4-16-year-old living in dengue-endemic countries (NCT02948829). Two hundred participants received TAK-003 on Days 1 and 90. Interferon-gamma (IFN-γ) enzyme-linked immunospot assay [ELISPOT] and intracellular cytokine staining were used to analyze T cell response and functionality, using peptide pools representing non-structural (NS) proteins NS3 and NS5 matching DENV-1, -2, -3, and -4 and DENV-2 NS1. One month after the second TAK-003 dose (Day 120), IFN-γ ELISPOT T cell response rates against any peptide pool were 97.1% (95% CI: 93.4% to 99.1%), and similar for baseline dengue seropositive (96.0%) and seronegative (98.6%) participants. IFN-γ ELISPOT T cell response rates at Day 120 were 79.8%, 90.2%, 77.3%, and 74.0%, against DENV-1, -2, -3, and -4, respectively, and remained elevated through 3 years post-vaccination. Multifunctional CD4 and CD8 T cell responses against DENV-2 NS peptides were observed, independent of baseline serostatus: CD8 T cells typically secreted IFN-γ and TNF-α whereas CD4 T cells secreted ≥ 2 of IFN-γ, IL-2 and TNF-α cytokines. NAb titers and seropositivity rates remained substantially elevated through 3 years post-vaccination. Overall, TAK-003 was well tolerated and elicited durable T cell responses against all four DENV serotypes irrespective of baseline serostatus in children and adolescents aged 4-16 years living in dengue-endemic countries. TAK-003-elicited CD4 and CD8 T cells were multifunctional and persisted up to 3 years post-vaccination.
© 2024. The Author(s).
Conflict of interest statement
The Authors declare the following competing financial and non-financial interests: S.M., S.B., A.F., I.E., D.F., N.R., N.F., M.S., M.B., and V.T. are employees of Takeda and may hold stock/stock options in Takeda. H.F. and J.R.C. received funds from Takeda for T-cell testing through a Cooperative Research and Development Agreement (CRADA) between Takeda Vaccines and WRAIR. C.B.-T., X.S.-L., R.G., J.A.L.D., R.D.A., and N.M. received institutional research funds from Takeda for study-related activities. Material has been reviewed by the Walter Reed Army Institute of Research. There is no objection to its presentation and/or publication. The opinions or assertions contained herein are the private views of the authors, and are not to be construed as official, or as reflecting true views of the Department of the Army or the Department of Defense. The investigators have adhered to the policies for the protection of human subjects as prescribed in AR 70–25.
Figures




References
-
- World Health Organization. Dengue and Severe Dengue (WHO, accessed 22 August 2023); https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue.
-
- Centers for Disease Control and Prevention. CDC Yellow Book—Dengue (CDC, accessed 19 September 2023); https://wwwnc.cdc.gov/travel/yellowbook/2024/infections-diseases/dengue (2024).
-
- European Centre for Disease Prevention and Control. Factsheet About Dengue (accessed 19 September 2023); https://www.ecdc.europa.eu/en/dengue-fever/facts (2023).
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

