MINCLE and TLR9 agonists synergize to induce Th1/Th17 vaccine memory and mucosal recall in mice and non-human primates
- PMID: 39420177
- PMCID: PMC11487054
- DOI: 10.1038/s41467-024-52863-9
MINCLE and TLR9 agonists synergize to induce Th1/Th17 vaccine memory and mucosal recall in mice and non-human primates
Abstract
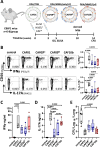
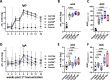
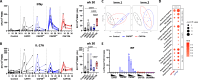
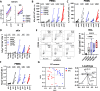
Development of new vaccines tailored for difficult-to-target diseases is hampered by a lack of diverse adjuvants for human use, and none of the currently available adjuvants induce Th17 cells. Here, we develop a liposomal adjuvant, CAF®10b, that incorporates Mincle and Toll-like receptor 9 agonists. In parallel mouse and non-human primate studies comparing to CAF® adjuvants already in clinical trials, we report species-specific effects of adjuvant composition on the quality and magnitude of the responses. When combined with antigen, CAF®10b induces Th1 and Th17 responses and protection against a pulmonary infection with Mycobacterium tuberculosis in mice. In non-human primates, CAF®10b induces higher Th1 responses and robust Th17 responses detectable after six months, and systemic and pulmonary Th1 and Th17 recall responses, in a sterile model of local recall. Overall, CAF®10b drives robust memory antibody, Th1 and Th17 vaccine-responses via a non-mucosal immunization route across both rodent and primate species.
© 2024. The Author(s).
Conflict of interest statement
JSW, GKP, DC and RM are co-inventors of a patent covering the use of CAF®10b and derivatives. All rights have been assigned to Statens Serum Institut (SSI). DC was employed by SSI during the time of the study, but is now employed by Croda Pharma that, among others, develops and produces immunostimulators, adjuvants and delivery systems for vaccines. The remaining authors declare no competing interests.
Figures



References
-
- Wilk, M. M. et al. Lung CD4 Tissue-Resident Memory T Cells Mediate Adaptive Immunity Induced by Previous Infection of Mice with Bordetella pertussis. J. Immunol.199, 233–243 (2017). - PubMed
-
- Dijkman, K. et al. Prevention of tuberculosis infection and disease by local BCG in repeatedly exposed rhesus macaques. Nat. Med25, 255–262 (2019). - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

