Multimodal gradients of basal forebrain connectivity across the neocortex
- PMID: 39420185
- PMCID: PMC11487139
- DOI: 10.1038/s41467-024-53148-x
Multimodal gradients of basal forebrain connectivity across the neocortex
Abstract
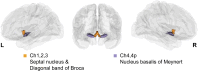
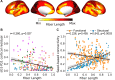
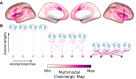
Cortical cholinergic projections originate from subregions of the basal forebrain (BF). To examine its organization in humans, we computed multimodal gradients of BF connectivity by combining 7 T diffusion and resting state functional MRI. Moving from anteromedial to posterolateral BF, we observe reduced tethering between structural and functional connectivity gradients, with the lowest tethering in the nucleus basalis of Meynert. In the neocortex, this gradient is expressed by progressively reduced tethering from unimodal sensory to transmodal cortex, with the lowest tethering in the midcingulo-insular network, and is also spatially correlated with the molecular concentration of VAChT, measured by [18F]fluoroethoxy-benzovesamicol (FEOBV) PET. In mice, viral tracing of BF cholinergic projections and [18F]FEOBV PET confirm a gradient of axonal arborization. Altogether, our findings reveal that BF cholinergic neurons vary in their branch complexity, with certain subpopulations exhibiting greater modularity and others greater diffusivity in the functional integration with their cortical targets.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





Update of
-
Multimodal gradients of basal forebrain connectivity across the neocortex.bioRxiv [Preprint]. 2024 May 25:2023.05.26.541324. doi: 10.1101/2023.05.26.541324. bioRxiv. 2024. Update in: Nat Commun. 2024 Oct 18;15(1):8990. doi: 10.1038/s41467-024-53148-x. PMID: 37292595 Free PMC article. Updated. Preprint.
References
-
- Mesulam, M. M., Mufson, E. J., Levey, A. I. & Wainer, B. H. Cholinergic innervation of cortex by the basal forebrain: cytochemistry and cortical connections of the septal area, diagonal band nuclei, nucleus basalis (substantia innominata), and hypothalamus in the rhesus monkey. J. Comp. Neurol.214, 170–197 (1983). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

