Paenilamicins are context-specific translocation inhibitors of protein synthesis
- PMID: 39420228
- PMCID: PMC11581978
- DOI: 10.1038/s41589-024-01752-9
Paenilamicins are context-specific translocation inhibitors of protein synthesis
Abstract
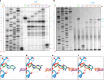
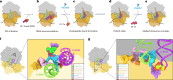
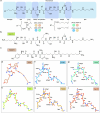
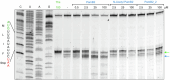
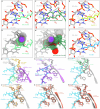
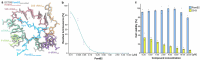
The paenilamicins are a group of hybrid nonribosomal peptide-polyketide compounds produced by the honey bee pathogen Paenibacillus larvae that display activity against Gram-positive pathogens, such as Staphylococcus aureus. While paenilamicins have been shown to inhibit protein synthesis, their mechanism of action has remained unclear. Here we determine structures of paenilamicin PamB2-stalled ribosomes, revealing a unique binding site on the small 30S subunit located between the A- and P-site transfer RNAs (tRNAs). In addition to providing a precise description of interactions of PamB2 with the ribosome, the structures also rationalize the resistance mechanisms used by P. larvae. We further demonstrate that PamB2 interferes with the translocation of messenger RNA and tRNAs through the ribosome during translation elongation, and that this inhibitory activity is influenced by the presence of modifications at position 37 of the A-site tRNA. Collectively, our study defines the paenilamicins as a class of context-specific translocation inhibitors.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures






References
-
- Müller, S. et al. Paenilamicin: structure and biosynthesis of a hybrid nonribosomal peptide/polyketide antibiotic from the bee pathogen Paenibacillus larvae. Angew. Chem. Int. Ed. Engl.53, 10821–10825 (2014). - PubMed
-
- Bulatov, T. et al. Total synthesis and biological evaluation of paenilamicins from the honey bee pathogen Paenibacillus larvae. J. Am. Chem. Soc.144, 288–296 (2022). - PubMed
-
- Genersch, E. American Foulbrood in honeybees and its causative agent, Paenibacillus larvae. J. Invertebr. Pathol.103, S10–S19 (2010). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

