Sex-specific phenotypical, functional and metabolic profiles of human term placenta macrophages
- PMID: 39420346
- PMCID: PMC11484421
- DOI: 10.1186/s13293-024-00652-w
Sex-specific phenotypical, functional and metabolic profiles of human term placenta macrophages
Abstract
Background: Placental macrophages, Hofbauer cells (HBC) are the only fetal immune cell population within the stroma of healthy placenta along pregnancy. They are central players in maintaining immune tolerance during pregnancy. Immunometabolism emerged a few years ago as a new field that integrates cellular metabolism with immune responses, however, the immunometabolism of HBC has not been explored yet. Here we studied the sex-specific differences in the phenotypic, functional and immunometabolic profile of HBC.
Methods: HBC were isolated from human term placentas (N = 31, 16 from male and 15 female neonates). Ex vivo assays were carried out to assess active metabolic and endoplasmic reticulum stress pathways by flow cytometry, confocal microscopy, gene expression and in silico approaches.
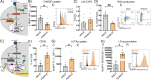
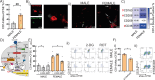
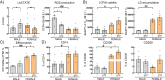
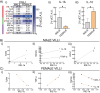
Results: HBC from female placentas displayed a stronger M2 phenotype accompanied by high rates of efferocytosis majorly sustained on lipid metabolism. On the other hand, male HBC expressed a weaker M2 phenotype with higher glycolytic metabolism. LPS stimulation reinforced the glycolytic metabolism in male but not in female HBC. Physiological endoplasmic reticulum stress activates IRE-1 differently, since its pharmacological inhibition increased lipid mobilization, accumulation and efferocytosis only in female HBC. Moreover, differential sex-associated pathways accompanying the phenotypic and functional profiles of HBC appeared related to the placental villi environment.
Conclusions: These results support sex-associated effects on the immunometabolism of the HBC and adds another layer of complexity to the intricate maternal-fetal immune interaction.
Keywords: Metabolism; Placental-macrophages; Sex-associated differences.
Plain language summary
Placental macrophages are the only fetal immune cell population within the stroma of healthy placenta along pregnancy and play a central role in contributing to the correct functioning of the placenta for the development of the fetus. Alterations in their metabolism lead to failures in their functions which are associated to pregnancy complications. Although, sex-specific differences were found in placental adaptation to pregnancy complications and outcomes, but the metabolism associated to their functions of placenta macrophages and whether they are associated to the sex of the placenta have not been explored so far. Here we studied human term placenta macrophages with special focus on their metabolism associated with their functions. We found out that macrophages from female placenta got energy from fatty acids whereas male macrophages used glucose. Furthermore, when female macrophages were exposed to a bacterial component, their metabolism or cellular function did not change towards one associated with a classic profile, but male macrophages did. These results might contribute to gain more insight into the immune-placental interactions at term human pregnancy and provide new clues to begin personalizing the pregnancy according to the sex of the fetus in physiological or pregnancy complications of inflammatory nature.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- O’Callaghan JL, Clifton VL, Prentis P, Ewing A, Saif Z, Pelzer ES. Sex-dependent differential transcript expression in the placenta of growth restricted infants. Placenta. 2022;128:1–8. - PubMed
-
- Sun T, Gonzalez TL, Deng N, DiPentino R, Clark EL, Lee B et al. Sexually Dimorphic Crosstalk at the Maternal-Fetal Interface. J Clin Endocrinol Metab [Internet]. 2020;105:e4831-47. http://www.ncbi.nlm.nih.gov/pubmed/32772088 - PMC - PubMed
MeSH terms
Grants and funding
- PICT 2020-2192/Agencia Nacional de Promoción Científica y Tecnológica
- PICT 2018-2584/Agencia Nacional de Promoción Científica y Tecnológica
- PICT 2021-I-A-00318/Agencia Nacional de Promoción Científica y Tecnológica
- PIBAA28720211010 1109CO/Consejo Nacional de Investigaciones Científicas y Tecnológicas (CONICET)
- 20020170100317BA/UBACyT
LinkOut - more resources
Full Text Sources

