Effectiveness and safety of insulin glargine 300 U/mL in insulin-naïve individuals according to diabetes duration: Results from the REALI European pooled data analysis
- PMID: 39420531
- PMCID: PMC11618222
- DOI: 10.1111/dom.16008
Effectiveness and safety of insulin glargine 300 U/mL in insulin-naïve individuals according to diabetes duration: Results from the REALI European pooled data analysis
Abstract
Aim: To evaluate the effectiveness and safety of insulin glargine 300 U/mL (Gla-300) initiation according to diabetes duration (DD).
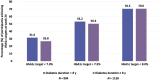
Materials and methods: We analysed patient-level data from 2381 insulin-naïve individuals with type 2 diabetes (T2D), of whom 2349 (98.7%) were treated with Gla-300 for 24 weeks. Of the 2381 participants, 1048 (44.0%) had a DD of less than 8 years and 1333 (56.0%) had a DD of 8 years or longer. We further analysed the subgroups of participants having a DD of less than 4 years (N = 450), 4-8 years (N = 598), 8-12 years (N = 627) and 12 years or longer (N = 706).
Results: Mean ± standard deviation age was 60.2 ± 9.0 years in participants with a DD less than 8 years and 64.2 ± 8.8 years in those with a DD of 8 years or longer. At 24 weeks of Gla-300 therapy, HbA1c improved with a least-squares (LS) mean change from baseline of -1.88% (95% confidence interval [CI], -1.95 to -1.80) and -1.71% (95% CI, -1.77 to -1.65), respectively, resulting in a LS mean difference between groups of 0.17% (95% CI, 0.07 to 0.26; P = .0005). In the subgroup analysis, LS mean HbA1c reduction from baseline to week 24 was highest in participants with a DD of less than 4 years and lowest in participants with a DD of 12 years or longer. Overall, incidences of symptomatic and severe hypoglycaemia were low, irrespective of DD, without body weight changes.
Conclusions: Gla-300 was effective and safe in insulin-naïve individuals with T2D, regardless of DD. Improvement in HbA1c was greater when Gla-300 was initiated in participants with a DD of less than 4 years, although the difference between the groups was modest.
Keywords: Gla‐300; diabetes duration; insulin glargine 300 U/mL; insulin‐naïve; type 2 diabetes.
© 2024 The Author(s). Diabetes, Obesity and Metabolism published by John Wiley & Sons Ltd.
Conflict of interest statement
DM‐W has acted as a consultant and has served on the speaker bureau for Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Daiichi‐Sankyo, Lilly, Merck Sharp & Dohme, Novo Nordisk and Sanofi. NF has received research support and has acted as a consultant for Gedeon Richter, Abbott Singapore, Galderma, ALK, AstraZeneca, Ipsen, Vertex, Sanofi, Thea, Aimmune, Novartis, Novo Nordisk, Allergan, Alliance and Merck Sharp & Dohme. RCB has served on the speaker bureau for Sanofi, Merck Sharp & Dohme, Bristol‐Myers Squibb, AstraZeneca and Janssen, and has served on the advisory panel for Merck Sharp & Dohme, Eli Lilly, Sanofi and Johnson & Johnson. CM is an IDDI employee and has acted as a biostatistics contractor for Sanofi. CV is an IVIDATA employee. MB is an employee and stakeholder of Sanofi. PG has received advisory board and speaker honoraria from Abbott, AbbVie, Amgen, AstraZeneca, Bayer, Novo Nordisk, Boehringer Ingelheim, Eli Lilly, Merck Sharp & Dohme, Mundipharma, Organon, Sanofi and Servier. DM has acted as a consultant and/or has served on the speaker bureau for Almirall, Eli Lilly, Esteve, Ferrer, Janssen, Menarini, Merck Sharp & Dohme, Novo Nordisk and Sanofi.
Figures


References
-
- Zoungas S, Woodward M, Li Q, et al. Impact of age, age at diagnosis and duration of diabetes on the risk of macrovascular and microvascular complications and death in type 2 diabetes. Diabetologia. 2014;57(12):2465‐2474. - PubMed
-
- Li FR, Yang HL, Zhou R, et al. Influence of diabetes duration and glycemic control on dementia: a cohort study. J Gerontol A Biol Sci Med Sci. 2021;76(11):2062‐2070. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

