RETINOBLASTOMA-RELATED Has Both Canonical and Noncanonical Regulatory Functions During Thermo-Morphogenic Responses in Arabidopsis Seedlings
- PMID: 39420660
- PMCID: PMC11695787
- DOI: 10.1111/pce.15202
RETINOBLASTOMA-RELATED Has Both Canonical and Noncanonical Regulatory Functions During Thermo-Morphogenic Responses in Arabidopsis Seedlings
Abstract
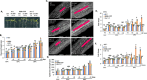
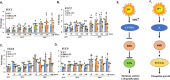
Warm temperatures accelerate plant growth, but the underlying molecular mechanism is not fully understood. Here, we show that increasing the temperature from 22°C to 28°C rapidly activates proliferation in the apical shoot and root meristems of wild-type Arabidopsis seedlings. We found that one of the central regulators of cell proliferation, the cell cycle inhibitor RETINOBLASTOMA-RELATED (RBR), is suppressed by warm temperatures. RBR became hyper-phosphorylated at a conserved CYCLIN-DEPENDENT KINASE (CDK) site in young seedlings growing at 28°C, in parallel with the stimulation of the expressions of the regulatory CYCLIN D/A subunits of CDK(s). Interestingly, while under warm temperatures ectopic RBR slowed down the acceleration of cell proliferation, it triggered elongation growth of post-mitotic cells in the hypocotyl. In agreement, the central regulatory genes of thermomorphogenic response, including PIF4 and PIF7, as well as their downstream auxin biosynthetic YUCCA genes (YUC1-2 and YUC8-9) were all up-regulated in the ectopic RBR expressing line but down-regulated in a mutant line with reduced RBR level. We suggest that RBR has both canonical and non-canonical functions under warm temperatures to control proliferative and elongation growth, respectively.
Keywords: cell cycle; elongation growth; retinoblastoma‐related; thermomorphogenesis.
© 2024 The Author(s). Plant, Cell & Environment published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Anderson, R. , Bayer P. E., and Edwards D.. 2020. “Climate Change and the Need for Agricultural Adaptation.” Current Opinion in Plant Biology 56: 197–202. - PubMed
-
- Arnell, N. W. , Lowe J. A., Challinor A. J., and Osborn T. J.. 2019. “Global and Regional Impacts of Climate Change at Different Levels of Global Temperature Increase.” Climatic Change 155: 377–391.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

