Inflammation‑based prognostic markers in patients with advanced or recurrent gastric cancer treated with nivolumab: Tokushukai REAl‑world Data project 02 (TREAD 02)
- PMID: 39421231
- PMCID: PMC11484223
- DOI: 10.3892/mco.2024.2788
Inflammation‑based prognostic markers in patients with advanced or recurrent gastric cancer treated with nivolumab: Tokushukai REAl‑world Data project 02 (TREAD 02)
Abstract
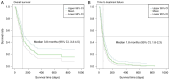
In addition to blood test data, inflammation-based prognostic markers have been used to predict the prognosis of various types of cancer. However, several of these previous studies may be outdated, as they were conducted prior to the widespread adoption of immune checkpoint inhibitors, leading to limited reports on their efficacy. The present study aimed to assess the accuracy of different inflammation-based prognostic markers in patients with advanced or recurrent gastric cancer undergoing nivolumab monotherapy as salvage-line chemotherapy. In a retrospective cohort study across Japan, a total of 159 patients with advanced or recurrent gastric cancer who were treated with nivolumab between September 2017 and March 2020 were selected. Blood test data were collected within 14 days of the start of chemotherapy and 17 inflammation-based prognostic markers were evaluated. Cox regression analysis was performed using all patient background factors. Subsequently, model selection was performed using backward elimination based on the Akaike information criterion (AIC) to obtain effective background factors which could be assessed for their impact on patient survival. For each marker, the magnitude of the impact on the survival rate, after adjusting for the background factors, was assessed using concordance and AIC analyses. A total of 159 patients (female, 30.2%; median age, 70 years) were included in the present study. Most patients received platinum, fluoropyrimidine and taxane treatment, with a median of three prior lines of systemic therapy. With a median follow-up of 3.3 months (95% CI, 2.5-3.8), median overall survival and time to treatment failure were 3.8 months (95% CI, 3.3-4.5) and 1.8 months (95% CI, 1.8-2.3), respectively. Amongst the 17 markers analyzed, the modified Glasgow prognostic score (mGPS) was classed as the most useful factor that affected the survival rate of patients. Real-world data showed that mGPS, an inflammation-based prognostic marker, had the strongest correlation with prognosis in patients with advanced or recurrent gastric cancer receiving nivolumab monotherapy. The present study was registered as a clinical trial with the UMIN Clinical Trial Registry (http://www.umin.ac.jp/ctr/index.htm) under the trial registration number UMIN000050590 on 15th March 2023.
Keywords: gastric cancer; inflammation-based prognostic markers; nivolumab; real-world data.
Copyright: © 2024 Shimoyama et al.
Conflict of interest statement
RS received speaker bureau fees/honoraria from Daiichi-Sankyo, Ono Pharm, Taiho Pharma and Chugai outside the scope of the submitted work. YI received speaker bureau fees/honoraria from Bayer, Bristol-Myers Squibb, Daiichi-Sankyo, Pfizer and Ono Pharm outside of the submitted work. HM received speaker bureau fees/honoraria from Daiichi-Sankyo and Ono Pharm, research funding from Astellas-Amgen Biopharma, Bayer, Bristol Myers Squibb, Chugai, Daiichi-Sankyo, Incite, Novartis, Ono Pharm, Pfizer and Rakuten Medical and scholarship donations from Bayer, Chugai, Daiichi-Sankyo, Eisai, Kyowa-Kirin, Lilly, Ono Pharmaceutical, Pfizer, Taiho Pharma and Takeda outside the scope of the submitted work. However, these organizations were not involved in the design, conduct or reporting of the present study.
Figures


References
-
- National Cancer Registry, Ministry of Health, Labour and Welfare Cancer Statistics: Cancer Information Service, National Cancer Center, Japan. https://ganjoho.jp/reg_stat/statistics/data/dl/index.html. Accessed on April 1, 2024.
-
- SEER 5-year relative survival rate, 2012-2018. Cancer Statistics Explorer Network, National Cancer Institute, United States. https://seer.cancer.gov/statistics-network/explorer/application.html. Accessed on April 1, 2024.
-
- NCCN Clinical Practice Guidelines in Oncology; Gastric Cancer Version 1.2024. https://www.nccn.org/guidelines/category_1. Accessed April 1, 2024.
LinkOut - more resources
Full Text Sources
