Toward Water-Resistant, Tunable Perovskite Absorbers Using Peptide Hydrogel Additives
- PMID: 39421276
- PMCID: PMC11480935
- DOI: 10.1021/acsaem.4c01089
Toward Water-Resistant, Tunable Perovskite Absorbers Using Peptide Hydrogel Additives
Abstract
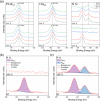
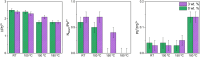
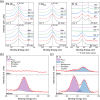
In recent years, hydrogels have been demonstrated as simple and cheap additives to improve the optical properties and material stability of organometal halide perovskites (OHPs), with most research centered on the use of hydrophilic, petrochemical-derived polymers. Here, we investigate the role of a peptide hydrogel in passivating defect sites and improving the stability of methylammonium lead iodide (MAPI, CH3NH3PbI3) using closely controlled, in situ X-ray photoelectron spectroscopy (XPS) techniques under realistic pressures. Optical measurements reveal that a reduction in the density of defect sites is achieved by incorporating peptide into the precursor solution during the conventional one-step MAPI fabrication approach. Increasing the concentration of peptide is shown to reduce the MAPI crystallite size, attributed to a reduction in hydrogel pore size, and a concomitant increase in the optical bandgap is shown to be consistent with that expected due to quantum size effects. Encapsulation of MAPI crystallites is further evidenced by XPS quantification, which demonstrates that the surface stoichiometry differs little from the expected nominal values for a homogeneously mixed system. In situ XPS demonstrates that thermally induced degradation in a vacuum is reduced by the inclusion of peptide, and near-ambient pressure XPS (NAP-XPS) reveals that this enhancement is partially retained at 9 mbar water vapor pressure, with a reduced loss of methylammonium (MA+) from the surface following heating achieved using 3 wt % peptide loading. A maximum power conversion efficiency (PCE) of 16.6% was achieved with a peptide loading of 3 wt %, compared with 15.9% from a 0 wt % device, the former maintaining 81% of its best efficiency over 480 h storage at 35% relative humidity (RH), compared with 48% maintained by a 0 wt % device.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures








References
-
- Leijtens T.; Bush K.; Cheacharoen R.; Beal R.; Bowring A.; McGehee M. D. Towards Enabling Stable Lead Halide Perovskite Solar Cells; Interplay between Structural, Environmental, and Thermal Stability. J. Mater. Chem. A 2017, 5 (23), 11483–11500. 10.1039/C7TA00434F. - DOI
-
- NREL . Best Research-Cell Efficiency Chart, 2024. https://www.nrel.gov/pv/cell-efficiency.html (accessed July 12, 2024).
LinkOut - more resources
Full Text Sources
Research Materials
