Case Report: Optimal utilization of marginal lung allografts by considering donor-recipient PGD risk compatibility and by mitigating allograft and recipient inflammatory risk
- PMID: 39421646
- PMCID: PMC11484051
- DOI: 10.3389/frtra.2024.1450376
Case Report: Optimal utilization of marginal lung allografts by considering donor-recipient PGD risk compatibility and by mitigating allograft and recipient inflammatory risk
Abstract
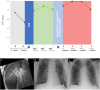
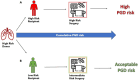
Reducing the risk of high-grade primary graft dysfunction (PGD) is vital to achieve acceptable short- and long-term outcomes for recipients following lung transplantation. However, the utilization of injured lung allografts, which may confer a higher risk of PGD, must be considered due to the disparity between the increasing number of patients requiring lung transplantation and the limited donor pool. We describe a case in which highly marginal lung allografts were utilized with a good post-transplant outcome. Donor-recipient PGD risk compatibility was taken into consideration. Normothermic ex vivo lung perfusion (EVLP) was utilized to functionally assess the allografts. A second cold ischemia time following EVLP was avoided by converting the EVLP mode to a hypothermic oxygenated perfusion setup from which the lungs were transplanted directly. We attempted to mitigate lung ischemia-reperfusion injury in the recipient by employing cytokine adsorption both during the EVLP and intraoperatively during the implant procedure. In this case report, we describe our hypothermic oxygenated perfusion setup on EVLP for the first time. Furthermore, we describe the utilization of cytokine adsorption in two phases of the same transplant process.
Keywords: EVLP; cytokine adsorption; hypothermic oxygenated lung perfusion; lung ischemia-reperfusion injury; lung transplant continuum; lung transplantation outcome; primary graft dysfunction.
© 2024 Braithwaite, Jennekens, Berg, de Heer, Ramjankhan, de Jong, Luc Charlier, Dessing, Veltkamp, Scheren, Ruigrok, Schönwetter, Buhre and van der Kaaij.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Snell GI, Yusen RD, Weill D, Strueber M, Garrity E, Reed A, et al. Report of the ISHLT working group on primary lung graft dysfunction, part I: definition and grading—a 2016 consensus group statement of the international society for heart and lung transplantation. J Heart Lung Transplant. (2017) 36(10):1097–103. 10.1016/j.healun.2017.07.021 - DOI - PubMed
-
- Van Raemdonck D, Hartwig MG, Hertz MI, Davis RD, Cypel M, Hayes Jr D, et al. Report of the ISHLT working group on primary lung graft dysfunction part IV: prevention and treatment: a 2016 consensus group statement of the international society for heart and lung transplantation. J Heart Lung Transplant. (2017) 36(10):1121–36. 10.1016/j.healun.2017.07.013 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

