Ex vivo optical coherence tomography combined with near infrared targeted fluorescence: towards in-vivo esophageal cancer detection
- PMID: 39421768
- PMCID: PMC11482167
- DOI: 10.1364/BOE.537828
Ex vivo optical coherence tomography combined with near infrared targeted fluorescence: towards in-vivo esophageal cancer detection
Abstract
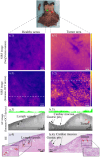
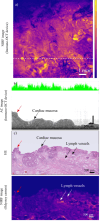
Early detection of (pre)malignant esophageal lesions is critical to improve esophageal cancer morbidity and mortality rates. In patients with advanced esophageal adenocarcinoma (EAC) who undergo neoadjuvant chemoradiation therapy, the efficacy of therapy could be optimized and unnecessary surgery prevented by the reliable assessment of residual tumors after therapy. Optical coherence tomography (OCT) provides structural images at a (sub)-cellular level and has the potential to visualize morphological changes in tissue. However, OCT lacks molecular imaging contrast, a feature that enables the study of biological processes at a cellular level and can enhance esophageal cancer diagnostic accuracy. We combined OCT with near-infrared fluorescence molecular imaging using fluorescently labelled antibodies (immuno-OCT). The main goal of this proof of principle study is to investigate the feasibility of immuno-OCT for esophageal cancer imaging. We aim to assess whether the sensitivity of our immuno-OCT device is sufficient to detect the tracer uptake using an imaging dose (∼100 times smaller than a dose with therapeutic effects) of a targeted fluorescent agent. The feasibility of immuno-OCT was demonstrated ex-vivo on dysplastic lesions resected from Barrett's patients and on esophageal specimens resected from patients with advanced EAC, who were respectively topically and intravenously administrated with the tracer bevacizumab-800CW. The detection sensitivity of our system (0.3 nM) is sufficient to detect increased tracer uptake with micrometer resolution using an imaging dose of labelled antibodies. Moreover, the absence of layered structures that are typical of normal esophageal tissue observed in OCT images of dysplastic/malignant esophageal lesions may further aid their detection. Based on our preliminary results, immuno-OCT could improve the detection of dysplastic esophageal lesions.
© 2024 The Author(s).
Conflict of interest statement
JFdB has IP licensed to Terumo, Heidelberg Engineering and ASML and receives royalties through his employer. The other authors declare that they have no conflict of interest.
Figures




References
LinkOut - more resources
Full Text Sources
