Shear-Sensing by C-Reactive Protein: Linking Aortic Stenosis and Inflammation
- PMID: 39421928
- PMCID: PMC11542976
- DOI: 10.1161/CIRCRESAHA.124.324248
Shear-Sensing by C-Reactive Protein: Linking Aortic Stenosis and Inflammation
Abstract
Background: CRP (C-reactive protein) is a prototypical acute phase reactant. Upon dissociation of the pentameric isoform (pCRP [pentameric CRP]) into its monomeric subunits (mCRP [monomeric CRP]), it exhibits prothrombotic and proinflammatory activity. Pathophysiological shear rates as observed in aortic valve stenosis (AS) can influence protein conformation and function as observed with vWF (von Willebrand factor). Given the proinflammatory function of dissociated CRP and the important role of inflammation in the pathogenesis of AS, we investigated whether shear stress can modify CRP conformation and induce inflammatory effects relevant to AS.
Methods: To determine the effects of pathological shear rates on the function of human CRP, pCRP was subjected to pathophysiologically relevant shear rates and analyzed using biophysical and biochemical methods. To investigate the effect of shear on CRP conformation in vivo, we used a mouse model of arterial stenosis. Levels of mCRP and pCRP were measured in patients with severe AS pre- and post-transcatheter aortic valve implantation, and the presence of CRP was investigated on excised valves from patients undergoing aortic valve replacement surgery for severe AS. Microfluidic models of AS were then used to recapitulate the shear rates of patients with AS and to investigate this shear-dependent dissociation of pCRP and its inflammatory function.
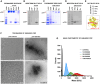
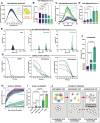
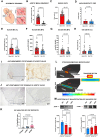
Results: Exposed to high shear rates, pCRP dissociates into its proinflammatory monomers (mCRP) and aggregates into large particles. Our in vitro findings were further confirmed in a mouse carotid artery stenosis model, where the administration of human pCRP led to the deposition of mCRP poststenosis. Patients undergoing transcatheter aortic valve implantation demonstrated significantly higher mCRP bound to circulating microvesicles pre-transcatheter aortic valve implantation compared with post-transcatheter aortic valve implantation. Excised human stenotic aortic valves display mCRP deposition. pCRP dissociated in a microfluidic model of AS and induces endothelial cell activation as measured by increased ICAM-1 (intercellular adhesion molecule 1) and P-selectin expression. mCRP also induces platelet activation and TGF-β (transforming growth factor beta) expression on platelets.
Conclusions: We identify a novel mechanism of shear-induced pCRP dissociation, which results in the activation of cells central to the development of AS. This novel mechanosensing mechanism of pCRP dissociation to mCRP is likely also relevant to other pathologies involving increased shear rates, such as in atherosclerotic and injured arteries.
Keywords: C-reactive protein; aortic valve stenosis; inflammation; proteolysis; thrombosis.
Conflict of interest statement
None.
Figures



References
-
- Baratchi S, Khoshmanesh K, Woodman OL, Potocnik S, Peter K, McIntyre P. Molecular sensors of blood flow in endothelial cells. Trends Mol Med. 2017;23:850–868. doi: 10.1016/j.molmed.2017.07.007 - PubMed
-
- Baratchi S, Zaldivia MTK, Wallert M, Loseff-Silver J, Al-Aryahi S, Zamani J, Thurgood P, Salim A, Htun NM, Stub D, et al. . Transcatheter aortic valve implantation represents an anti-inflammatory therapy via reduction of shear stress–induced, piezo-1–mediated monocyte activation. Circulation. 2020;142:1092–1105. doi: 10.1161/CIRCULATIONAHA.120.045536 - PubMed
-
- Malek AM, Alper SL, Izumo S. Hemodynamic shear stress and its role in atherosclerosis. JAMA. 1999;282:2035–2042. doi: 10.1001/jama.282.21.2035 - PubMed
-
- Ruggeri ZM, Platelets in atherothrombosis. Nat Med. 2002;8:1227–1234. doi: 10.1038/nm1102-1227 - PubMed
-
- Pareti FI, Lattuada A, Bressi C, Zanobini M, Sala A, Steffan A, Ruggeri ZM. Proteolysis of von Willebrand factor and shear stress–induced platelet aggregation in patients with aortic valve stenosis. Circulation. 2000;102:1290–1295. doi: 10.1161/01.cir.102.11.1290 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

