Metabolic Effects of the SGLT2 Inhibitor Dapagliflozin in Heart Failure Across the Spectrum of Ejection Fraction
- PMID: 39421941
- PMCID: PMC11634023
- DOI: 10.1161/CIRCHEARTFAILURE.124.011980
Metabolic Effects of the SGLT2 Inhibitor Dapagliflozin in Heart Failure Across the Spectrum of Ejection Fraction
Abstract
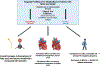
Background: Mechanisms of benefit with SGLT2is (sodium-glucose cotransporter-2 inhibitors) in heart failure (HF) remain incompletely characterized. Dapagliflozin alters ketone and fatty acid metabolism in HF with reduced ejection fraction though similar effects have not been observed in HF with preserved ejection fraction. We explore whether metabolic effects of SGLT2is vary across the left ventricular ejection fraction spectrum and their relationship with cardiometabolic end points in 2 randomized trials of dapagliflozin in HF.
Methods: Metabolomic profiling of 61 metabolites was performed in 527 participants from DEFINE-HF (Dapagliflozin Effects on Biomarkers, Symptoms and Functional Status in Patients With HF With Reduced Ejection Fraction) and PRESERVED-HF (Dapagliflozin in PRESERVED Ejection Fraction HF; 12-week, placebo-controlled trials of dapagliflozin in HF with reduced ejection fraction and HF with preserved ejection fraction, respectively). Linear regression was used to assess changes in principal components analysis-defined metabolite factors with treatment from baseline to 12 weeks, as well as the relationship between changes in metabolite clusters and HF-related end points.
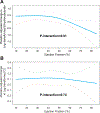
Results: The mean age was 66±11 years, 43% were female, and 33% were self-identified as Black. Two principal components analysis-derived metabolite factors (which were comprised of ketone and short-/medium-chain acylcarnitines) increased with dapagliflozin compared with placebo. Ketosis (defined as 3-hydroxybutyrate >500 μM) was achieved in 4.5% with dapagliflozin versus 1.2% with placebo (P=0.03). There were no appreciable treatment effects on amino acids, including branched-chain amino acids. Increases in several acylcarnitines were consistent across LVEF (Pinteraction>0.10), whereas the ketogenic effect diminished at higher LVEF (Pinteraction=0.01 for 3-hydroxybutyrate). Increases in metabolites reflecting mitochondrial dysfunction (particularly long-chain acylcarnitines) and aromatic amino acids and decreases in branched-chain amino acids were associated with worse HF-related outcomes in the overall cohort, with consistency across treatment and LVEF.
Conclusions: SGLT2is demonstrate common (fatty acid) and distinct (ketogenic) metabolic signatures across the LVEF spectrum. Changes in key pathways related to fatty acid and amino acid metabolism are associated with HF-related end points and may serve as therapeutic targets across HF subtypes.
Registration: URL: https://www.clinicaltrials.gov; Unique Identifiers: NCT03030235 and NCT02653482.
Keywords: fatty acid; heart failure; ketone bodies; metabolomics; quality of life.
Conflict of interest statement
Dr Selvaraj receives research support from the National Heart, Lung, and Blood Institute (grant K23HL161348), the Doris Duke Foundation (grant 2020061), the American Heart Association (grant 935275), the Mandel Foundation, the Duke Heart Center Leadership Council, the Institute for Translational Medicine and Therapeutics, and the Foundation for Sarcoidosis Research. Dr Selvaraj has participated in advisory boards for AstraZeneca for unrelated work. Dr Sauer performs consulting and advising or receives research funding from Abbott, Boston Scientific, Biotronik, Bayer, Amgen, CSL Vifor, AstraZeneca, Acorai, Story Health, FIRE1, uLink, General Prognostics, Impulse Dynamics, Edwards Lifesciences, 35Pharma, Rivus, and Pfizer, and he owns stock as a senior advisor to ISHI, a private digital health company. Dr McGarrah has been a consultant for AstraZeneca and M3 and received research funding from Eli Lilly. Dr Newgard is a member of the Eli Lilly Global Diabetes Advisory Board. Dr Borlaug receives research support from the National Institutes of Health (NIH) and the US Department of Defense and research grant funding from AstraZeneca, Axon, GlaxoSmithKline, Medtronic, Mesoblast, Novo Nordisk, and Tenax Therapeutics. Dr Borlaug has served as a consultant for Actelion, Amgen, Aria, BD, Boehringer Ingelheim, Cytokinetics, Edwards Lifesciences, Eli Lilly, Janssen, Merck, and Novo Nordisk. Dr Borlaug and Shah are named inventors (US Patent 10,307,179) for the tools and approach for a minimally invasive pericardial modification procedure to treat heart failure. Dr Kitzman has been a consultant for AstraZeneca, Pfizer, Corvia Medical, Bayer, Boehringer Ingelheim, Novo Nordisk, Rivus, and St. Luke’s Medical Center; received grant support from Novartis, AstraZeneca, Bayer, Pfizer, Novo Nordisk, Rivus, and St. Luke’s Medical Center; and owns stock in Gilead Sciences. Dr Sanjiv Shah reports support from research grants from the NIH (U54 HL160273, R01 HL140731, and R01 HL149423), Pfizer, and AstraZeneca and consulting fees from Abbott, Alleviant, AstraZeneca, Amgen, Aria CV, Axon Therapies, Bayer, Boehringer Ingelheim, Boston Scientific, Bristol Myers Squibb, Cyclerion, Corvia, Cytokinetics, Edwards Lifesciences, Eidos, Imara, Impulse Dynamics, Intellia, Ionis, Lilly, Merck, Metabolic Flux, MyoKardia, NGM Biopharmaceuticals, Novartis, Novo Nordisk, Pfizer, Prothena, Regeneron, Rivus, Sardocor, Shifamed, Tenax, Tenaya, Ultromics, and United Therapeutics. Dr Margulies reports sponsored research funding from Amgen, Bristol Myers Squibb, and Lexicon Pharmaceuticals and advisory board activities for Amgen and Bristol Myers Squibb. Dr David Lanfear reports support from NIH grants (P50MD017351 and R01HL132154) and Illumina; consulting fees from Janssen, AstraZeneca, and Abbot Laboratories (via ACI clinical); and clinical trial participation with Lilly, Pfizer, AstraZeneca, Bayer, Illumina, and Janssen. Dr Kosiborod reports research grant support from AstraZeneca, Boehringer Ingelheim, and Pfizer; is on the consultant/advisory board of 35Pharma, Alnylam, Amgen, Applied Therapeutics, AstraZeneca, Bayer, Boehringer Ingelheim, Cytokinetics, Dexcom, Eli Lilly, Esperion Therapeutics, Imbria Pharmaceuticals, Janssen, Lexicon Pharmaceuticals, Merck (Diabetes and Cardiovascular), Novo Nordisk, Pharmacosmos, Pfizer, Sanofi, scPharmaceuticals, Structure Therapeutics, Vifor Pharma, and Youngene Therapeutics; other research support from AstraZeneca; honoraria from AstraZeneca, Boehringer Ingelheim, and Novo Nordisk; and stock options in Artera Health and Saghmos Therapeutics. Dr Svati Shah reports research funding through sponsored research agreements to Duke University from AstraZeneca, Lilly Inc, Verily Inc, and nference and is a co-inventor of unlicensed patents held by Duke University. The other authors report no conflicts.
Figures


Comment in
-
SGLT2 Inhibitors and Their Effect on Metabolism in Patients With Heart Failure.Circ Heart Fail. 2024 Nov;17(11):e012373. doi: 10.1161/CIRCHEARTFAILURE.124.012373. Epub 2024 Oct 18. Circ Heart Fail. 2024. PMID: 39421945 No abstract available.
References
-
- Vaduganathan M, Docherty KF, Claggett BL, Jhund PS, de Boer RA, Hernandez AF, Inzucchi SE, Kosiborod MN, Lam CSP, Martinez F, et al. Sglt-2 inhibitors in patients with heart failure: A comprehensive meta-analysis of five randomised controlled trials. Lancet. 2022;400:757–767 - PubMed
-
- Pandey AK, Bhatt DL, Pandey A, Marx N, Cosentino F, Pandey A, Verma S. Mechanisms of benefits of sodium-glucose cotransporter 2 inhibitors in heart failure with preserved ejection fraction. Eur Heart J. 2023;44:3640–3651 - PubMed
MeSH terms
Substances
Associated data
Grants and funding
- R01 HL162828/HL/NHLBI NIH HHS/United States
- R01 AG078153/AG/NIA NIH HHS/United States
- R01 HL140731/HL/NHLBI NIH HHS/United States
- R01 AG018915/AG/NIA NIH HHS/United States
- P50 MD017351/MD/NIMHD NIH HHS/United States
- R01 HL149423/HL/NHLBI NIH HHS/United States
- R01 HL160689/HL/NHLBI NIH HHS/United States
- P30 DK124723/DK/NIDDK NIH HHS/United States
- P30 AG021332/AG/NIA NIH HHS/United States
- R01 HL128526/HL/NHLBI NIH HHS/United States
- K23 HL161348/HL/NHLBI NIH HHS/United States
- R01 HL132154/HL/NHLBI NIH HHS/United States
- U24 AG059624/AG/NIA NIH HHS/United States
- R01 AG045551/AG/NIA NIH HHS/United States
- U54 HL160273/HL/NHLBI NIH HHS/United States
- U01 HL160272/HL/NHLBI NIH HHS/United States
- U01 HL160226/HL/NHLBI NIH HHS/United States
- U01 AG076928/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

