Irisin improves ROS‑induced mitohormesis imbalance in H9c2 cells
- PMID: 39422020
- PMCID: PMC11544398
- DOI: 10.3892/mmr.2024.13364
Irisin improves ROS‑induced mitohormesis imbalance in H9c2 cells
Abstract
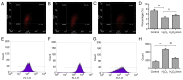
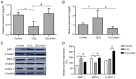
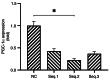
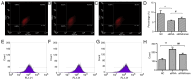
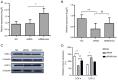
Abnormal mitohormesis is a key pathogenic mechanism that induces a variety of cardiac diseases, including cardiac hypertrophy and heart failure. Irisin as a muscle factor serves a cardioprotective role in response to cellular oxidative stress injury. Rat cardiomyocyte cells (H9c2) were treated with 40 µM exogenous H2O2 to establish an oxidative stress model, followed by addition of 75 nM exogenous irisin for experiments to determine mitochondrial membrane potential, reactive oxygen species, and Mitohormesis‑related factors by attrition cytometry. Subsequently, the expression of mitochondrial membrane potential, reactive oxygen species and Mitohormesis‑related factors were continued to be determined by establishing a peroxisome proliferator‑activated receptor γ coactivator‑1 alpha (PGC‑1α) siRNA interference model and continuing the treatment with the addition of 75 nM irisin 12 h before the end of interference. When H9c2 cells underwent oxidative stress, irisin partially improved mitochondrial membrane potential and reactive oxygen species levels and partially restored mitochondrial energy metabolism by upregulating fusion proteins optic atrophy 1 (OPA1) mitochondrial dynamin‑like GTPase and mitofusin 2 and downregulating fission protein dynamin‑related protein 1. Following interference with PGC‑1α, irisin promoted mitochondrial biosynthesis by increasing the mRNA levels of OPA1 and protein levels of cytochrome c oxidase subunit 4. These results suggested that irisin acted partially independently of the PGC‑1α signaling pathway to regulate mitohormesis imbalance due to oxidative stress and maintain energy metabolism by improving mitochondrial structure.
Keywords: H9c2; PGC‑1α; irisin; mitohormesis; reactive oxygen species.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
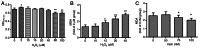
Similar articles
-
Melatonin elicits protective effects on OGD/R‑insulted H9c2 cells by activating PGC‑1α/Nrf2 signaling.Int J Mol Med. 2020 May;45(5):1294-1304. doi: 10.3892/ijmm.2020.4514. Epub 2020 Feb 25. Int J Mol Med. 2020. PMID: 32323734 Free PMC article.
-
Metformin protects high glucose‑cultured cardiomyocytes from oxidative stress by promoting NDUFA13 expression and mitochondrial biogenesis via the AMPK signaling pathway.Mol Med Rep. 2020 Dec;22(6):5262-5270. doi: 10.3892/mmr.2020.11599. Epub 2020 Oct 14. Mol Med Rep. 2020. PMID: 33174032 Free PMC article.
-
Irisin Attenuates Oxidative Stress, Mitochondrial Dysfunction, and Apoptosis in the H9C2 Cellular Model of Septic Cardiomyopathy through Augmenting Fundc1-Dependent Mitophagy.Oxid Med Cell Longev. 2021 Aug 18;2021:2989974. doi: 10.1155/2021/2989974. eCollection 2021. Oxid Med Cell Longev. 2021. Retraction in: Oxid Med Cell Longev. 2023 Oct 11;2023:9790868. doi: 10.1155/2023/9790868. PMID: 34457111 Free PMC article. Retracted.
-
Inhibition of miR-23a attenuates doxorubicin-induced mitochondria-dependent cardiomyocyte apoptosis by targeting the PGC-1α/Drp1 pathway.Toxicol Appl Pharmacol. 2019 Apr 15;369:73-81. doi: 10.1016/j.taap.2019.02.016. Epub 2019 Mar 1. Toxicol Appl Pharmacol. 2019. PMID: 30831132
-
Peroxisome proliferator‑activated receptor γ coactivator‑1α in heart disease (Review).Mol Med Rep. 2025 Jan;31(1):17. doi: 10.3892/mmr.2024.13382. Epub 2024 Nov 8. Mol Med Rep. 2025. PMID: 39513608 Free PMC article. Review.
Cited by
-
Notoginsenoside R1 Attenuates H/R Injury in H9c2 Cells by Maintaining Mitochondrial Homeostasis.Curr Issues Mol Biol. 2025 Jan 10;47(1):44. doi: 10.3390/cimb47010044. Curr Issues Mol Biol. 2025. PMID: 39852159 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

