Switching biologics in chronic rhinosinusitis with nasal polyps: A multicenter Canadian experience
- PMID: 39422074
- PMCID: PMC11785148
- DOI: 10.1002/alr.23466
Switching biologics in chronic rhinosinusitis with nasal polyps: A multicenter Canadian experience
Abstract
Background: Type 2 biologics have been used increasingly for the treatment of chronic rhinosinusitis with nasal polyps (CRSwNP). However, patterns of biologic switching are understudied, and established guidelines for sequential or simultaneous use do not yet exist.
Methods: This is a Canadian multicenter retrospective study of real-world patient data. Patients were included if they had recurrent CRSwNP despite maximal medical and surgical management, and received at least one dose of a type 2 biologic. Patients who remained on their initial biologic comprised the continuous group. Patients with sequential or simultaneous use of more than one biologic comprised the switched group. We compared the characteristics of patients who continued and switched biologics.
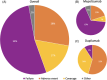
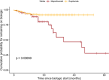
Results: Note that 225 consecutive patients were included. Thirty-six (16%) switched biologics at least once, and six (3%) switched twice. The most common switch was from mepolizumab to dupilumab, with poor control of CRSwNP symptoms being the leading cause for this switch. Lack of efficacy was the main reason for switching off mepolizumab and omalizumab, while adverse events were the leading cause for switching off dupilumab. Additionally, mepolizumab patients were more likely to switch biologics late in their treatment, while dupilumab patients rarely switched after 12 months of therapy (p-value < 0.001).
Conclusions: Switching biologics for CRSwNP is frequent in Canadian rhinology practices, with 16% of patients switching at least once. The most common switch is from mepolizumab to dupilumab with inadequate CRSwNP control driving this switch. This study may help guide sequential or simultaneous use of biologics in CRSwNP patients.
Keywords: asthma; biologics; chronic rhinosinusitis; chronic rhinosinusitis with nasal polyposis; lower airway disease; type 2 inflammation; upper airway disease.
© 2024 The Author(s). International Forum of Allergy & Rhinology published by Wiley Periodicals LLC on behalf of American Academy of Otolaryngic Allergy and American Rhinologic Society.
Conflict of interest statement
Juan Carlos Hernaiz‐Leonardo: Speaking for Glaxo‐Smith‐Kline. Arif Janjua: Research, speaking, and consulting: Glaxo‐Smith‐Kline, Sanofi, and Astra‐Zeneca.
Amin Javer: Research, speaking, and consulting: Glaxo‐Smith‐Kline, Sanofi, and Astra‐Zeneca.
Doron Sommer: Research, advising, and educational support: Glaxo‐Smith‐Kline and Sanofi. Speaking: Medtronic, Stryker, and Miravo. Consulting: Stryker. John Lee: Advising and speaking: Glaxo‐Smith‐Kline, Sanofi, and Regeneron. Yvonne Chan: Advisory Boards: Glaxo‐Smith‐Kline, Sanofi, and Medtronic. Speaker's Bureau: Medtronic, Glaxo‐Smith‐Kline, Sanofi, and Stryker. Andrew Thamboo: Research, speaking, and consulting: Glaxo‐Smith‐Kline, Sanofi, and Astra‐Zeneca. The remaining authors have no conflicts of interest.
Figures

References
-
- Papacharalampous GX, Constantinidis J, Fotiadis G, et al. Chronic rhinosinusitis with nasal polyps (CRSwNP) treated with omalizumab, dupilumab, or mepolizumab: a systematic review of the current knowledge towards an attempt to compare agents' efficacy. Int Forum Allergy Rhinol. 2024;14(1):96‐109. doi: 10.1002/alr.23234 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

