Faecal Volatile Organic Compounds to Detect Colorectal Neoplasia in Lynch Syndrome-A Prospective Longitudinal Multicentre Study
- PMID: 39422092
- PMCID: PMC11636173
- DOI: 10.1111/apt.18328
Faecal Volatile Organic Compounds to Detect Colorectal Neoplasia in Lynch Syndrome-A Prospective Longitudinal Multicentre Study
Abstract
Background: Non-invasive biomarkers may reduce post-colonoscopy colorectal cancer (CRC) rates and colonoscopy overuse in Lynch syndrome. Unlike faecal immunochemical test (FIT), faecal volatile organic compounds (VOCs) may accurately detect both advanced and non-advanced colorectal neoplasia.
Aim: The aim of this study was to evaluate the potential of faecal VOCs-separately and with FIT-to guide optimal colonoscopy intervals in Lynch syndrome.
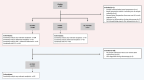
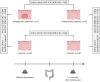
Methods: Prospective longitudinal multicentre study in which individuals with Lynch syndrome collected faeces before and after high-quality surveillance colonoscopy. VOC-patterns were analysed using field asymmetric ion mobility spectrometry (FAIMS) and gas chromatography-ion mobility spectrometry (GC-IMS) followed by machine learning pipelines, and combined with FIT at 2.55 μg Hb/g faeces. Gas chromatography time-of-flight mass spectrometry analysed individual VOC abundance.
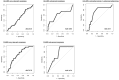
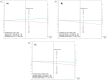
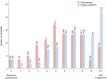
Results: Among 200 included individuals (57% female, median 51 years), 62 had relevant neoplasia at colonoscopy: 3 CRC, 6 advanced adenoma (AA), 3 advanced serrated lesion (ASL), and 50 non-advanced adenoma (NAA). Respective sensitivity and negative predictive value for CRC and AA (and also ASL in case of FAIMS) were 100% and 100% using FAIMS (54% specificity), and 89% and 99% using GC-IMS (58% specificity). Respective sensitivity and specificity for any relevant neoplasia were 88% and 44% (FAIMS) and 84% and 28% (GC-IMS); accuracy did not significantly improve upon VOC-FIT. VOC-patterns differed before and after polypectomy (AUC 0.70). NAA showed decreased faecal abundance of butanal, 2-oxohexane, dimethyldisulphide and dimethyltrisulphide.
Conclusions: In Lynch syndrome, faecal VOCs may be a promising strategy for postponing colonoscopy and for follow-up after polypectomy. Our results serve as a stepping stone for large validation studies.
Trial registration: NL8749.
Keywords: Lynch syndrome; biomarkers; colorectal cancer; faecal immunochemical test; surveillance; volatile organic compounds.
© 2024 The Author(s). Alimentary Pharmacology & Therapeutics published by John Wiley & Sons Ltd.
Conflict of interest statement
E.L.S.A.L., Emma D., M.D., J.M.L., T.K.S., S.B., M.A.J.M.J., J.J.K., J.P.K., M.E.L. and J.A.C. declare no competing interests. D.R. has received a research grant (unrestricted) from AbbVie. He has served as a member of the data safety monitoring board of the VIVIAD trial. B.C. has several patents pending and/or issued. Evelien D. has endoscopic equipment on a loan from FujiFilm and has received a research grant from FujiFilm. She has received an honorarium for a consultancy from FujiFilm, Olympus, InterVenn and Ambu, and speakers' fees from Olympus, GI Supply, Norgine, IPSEN, PAION and FujiFilm. T.G.J.M. has served as a speaker for Nutricia, Mead Johnson and Winclove. He has served as an advisory board member for Nutricia. G.A.M. is cofounder and board member (CSO) of CRCbioscreen BV. He has a research collaboration with CZ Health Insurances (cash matching to ZonMW grant) and with Exact Sciences, Sysmex, Sentinel Ch. SpA, Personal Genome Diagnostics (PGDX), DELFi and Hartwig Medical Foundation; these companies provide materials, equipment, and/or sample/genomic analyses. G.A.M. is an Advisory Board member of ‘Missie Tumor Onbekend’. M.C.W.S. has received research support from Sysmex, Sentinel, Medtronic and Norgine. N.K.H.B. has served as a speaker for AbbVie and MSD and has served as a consultant and principal investigator for TEVA Pharma BV and Takeda. He has received a research grant (unrestricted) from Dr. Falk, TEVA Pharma BV, Dutch Digestive Foundation (MLDS) and Takeda.
Figures
References
-
- van Leerdam M. E., Roos V. H., van Hooft J. E., et al., “Endoscopic Management of Lynch Syndrome and of Familial Risk of Colorectal Cancer: European Society of Gastrointestinal Endoscopy (ESGE) Guideline,” Endoscopy 51, no. 11 (2019): 1082–1093. - PubMed
-
- Vasen H., Hes F., and de Jong M., “Dutch Guideline for Diagnostics and Prevention of Hereditary and Familial Tumours,” 2017, https://www.stoet.nl/wp‐content/uploads/2017/04/STOET‐Richtlijnenboekje‐....
-
- Denters M. J., Schreuder M., Depla A. C., et al., “Patients' Perception of Colonoscopy: Patients With Inflammatory Bowel Disease and Irritable Bowel Syndrome Experience the Largest Burden,” European Journal of Gastroenterology & Hepatology 25, no. 8 (2013): 964–972. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
