Harnessing Lactobacillus reuteri-Derived Extracellular Vesicles for Multifaceted Cancer Treatment
- PMID: 39422169
- PMCID: PMC11899519
- DOI: 10.1002/smll.202406094
Harnessing Lactobacillus reuteri-Derived Extracellular Vesicles for Multifaceted Cancer Treatment
Abstract
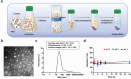
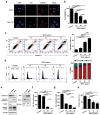
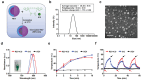
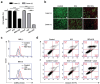
Extracellular vesicles (EVs) have emerged as valuable biological materials for treating intractable diseases. Extensive studies are conducted on EVs derived from various cellular sources. In this study, EVs derived from Lactobacillus reuteri (L. reuteri), a probiotic, exhibit remarkable cancer therapeutic efficacy when administered orally is reported. These L. reuteri-derived EVs (REVs) demonstrate stability in the gastrointestinal tract and exert significant anti-tumor effects. Using A549 cells and murine models, we confirmed that REVs mediate their therapeutic effects by modulating apoptotic signaling pathways. Furthermore, the combination of REV with drugs enhances tumor ablation and induces immunogenic cell death. In a mouse model, oral administration of REVs encapsulating indocyanine green followed by photothermal therapy led to complete tumor elimination within 32 days. REVs represent a promising biological therapeutic platform for cancer treatment, either independently or in combination with other therapies, depending on the treatment objectives.
Keywords: drug delivery platforms; extracellular vesicles; oral administration; photothermal therapy; probiotics.
© 2024 The Author(s). Small published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- a) Kolonics F., Szeifert V., Timar C. I., Ligeti E., Lorincz A. M., Cells 2020, 9; - PMC - PubMed
- b) Hallal S., Tuzesi A., Grau G. E., Buckland M. E., Alexander K. L., J. Extracell. Vesicles 2022, 11, e12260; - PMC - PubMed
- c) Ghadami S., Dellinger K., Front. Mol. Biosci. 2023, 10, 1198044. - PMC - PubMed
-
- a) Zaborowski M. P., Balaj L., Breakefield X. O., Lai C. P., Bioscience 2015, 65, 783; - PMC - PubMed
- b) Gurung S., Perocheau D., Touramanidou L., Baruteau J., Cell Commun. Signal 2021, 19, 47; - PMC - PubMed
- c) Hao Y., Song H., Zhou Z., Chen X., Li H., Zhang Y., Wang J., Ren X., Wang X., J. Control Rel. 2021, 340, 136; - PubMed
- d) Chen C., Zheng H., Luo Y., Kong Y., An M., Li Y., He W., Gao B., Zhao Y., Huang H., Huang J., Lin T., J. Clin. Invest. 2021, 131. - PMC - PubMed
-
- a) Jong A. Y., Wu C. H., Li J., Sun J., Fabbri M., Wayne A. S., Seeger R. C., J. Extracell Vesicles 2017, 6, 1294368; - PMC - PubMed
- b) Zhu L., Gangadaran P., Kalimuthu S., Oh J. M., Baek S. H., Jeong S. Y., Lee S. W., Lee J., Ahn B. C., Artif. Cells Nanomed. Biotechnol. 2018, 46, 166; - PubMed
- c) Di Pace A. L., Tumino N., Besi F., Alicata C., Conti L. A., Munari E., Maggi E., Vacca P., Moretta L., Cancers 2020, 12; - PMC - PubMed
- d) Choi J. W., Lim S., Kang J. H., Hwang S. H., Hwang K. C., Kim S. W., Lee S., Molecules 2020, 25; - PMC - PubMed
- e) Zhu L., Kalimuthu S., Gangadaran P., Oh J. M., Lee H. W., Baek S. H., Jeong S. Y., Lee S. W., Lee J., Ahn B. C., Theranostics 2017, 7, 2732. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
- KGM5472413/Korea Research Institute of Bioscience and Biotechnology
- National NanoFab Center
- RS-2024-00401639/Ministry of Agriculture, Food and Rural Affairs
- 2021003370003/Ministry of Environment
- RS-2022-00154853/Ministry of Trade, Industry and Energy
- RS-2024-00403563/Ministry of Trade, Industry and Energy
- RS-2024-00432382/Ministry of Trade, Industry and Energy
- 2021M3H4A1A02051048/Ministry of Science and ICT, South Korea
- 2023R1A2C2005185/Ministry of Science and ICT, South Korea
- 2021M3E5E3080844/Ministry of Science and ICT, South Korea
- 2022R1C1C1008815/Ministry of Science and ICT, South Korea
- RS-2024-00348576/Ministry of Science and ICT, South Korea
- RS-2024-00438316/Ministry of Science and ICT, South Korea
- RS-2024-00459749/Ministry of Science and ICT, South Korea
LinkOut - more resources
Full Text Sources
Medical

