Effectiveness of tolvaptan on renal replacement therapy in patients with autosomal dominant polycystic kidney disease: a retrospective cohort study from the TriNetX global collaborative network
- PMID: 39422218
- PMCID: PMC11492389
- DOI: 10.1080/0886022X.2024.2412721
Effectiveness of tolvaptan on renal replacement therapy in patients with autosomal dominant polycystic kidney disease: a retrospective cohort study from the TriNetX global collaborative network
Abstract
Background and hypothesis: Autosomal Dominant Polycystic Kidney Disease (ADPKD) is a major genetic contributor to end-stage kidney disease (ESKD). Current evidence on tolvaptan primarily focuses on slowing estimated glomerular filtration rate (eGFR) decline and kidney volume growth. However, direct confirmation of its effectiveness in reducing the need for hemodialysis in ESKD remains limited.
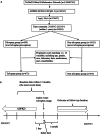
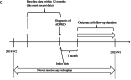
Methods: We included ADPKD patients aged ≥18 years using TriNetx data from Sep 2, 2018, to Sep 3, 2023. Propensity score matching (PSM) ensured baseline comparability (standardized mean difference (SMD) <0.1). Hazard ratios (HRs) with 95% confidence intervals (CIs) evaluated outcomes, and subgroup analyses were performed.
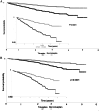
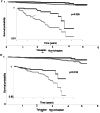
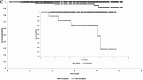
Results: After 1:1 PSM, both groups comprised 673 patients. The average age was 45, with generally good health (3-5% diabetes, 2-3% ischemic heart disease). Baseline eGFR averaged ∼55 ml/min/1.732m2. Post-matching, all SMDs were <0.1, indicating successful matching. Tolvaptan users exhibited lower eGFR (51.45 ± 30.09 vs. 57.37 ± 33.65, p < 0.001) and higher risk of stage 4-CKD (HR: 2.436, 95% CI:1.649, 3.599) compared to non-users. However, tolvaptan users showed significantly reduced chances of initiating hemodialysis (HR:0.362, 95%CI:0.176, 0.745), experiencing urinary tract infections (HR:0.581, 95%CI:0.354, 0.956), and all-cause mortality (HR:0.355, 95% CI:0.180, 0.700). Kaplan-Meier curves for hemodialysis initiation indicated higher survival rates among tolvaptan users across age and number of medication refill subgroups.
Conclusions: This real-world study, employing precise matching, reveals tolvaptan's role in reducing hemodialysis initiation risk in ADPKD, despite initial hemodynamic-induced lower eGFR.
Keywords: Tolvaptan; autosomal dominant polycystic kidney disease (ADPKD); end-stage kidney disease (ESKD); hemodialysis; mortality.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures

References
-
- Spithoven EM, Kramer A, Meijer E, et al. . Analysis of data from the ERA-EDTA Registry indicates that conventional treatments for chronic kidney disease do not reduce the need for renal replacement therapy in autosomal dominant polycystic kidney disease. Kidney Int. 2014;86(6):1244–1252. doi: 10.1038/ki.2014.120. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
