Safety and immunogenicity of a modified mRNA-lipid nanoparticle vaccine candidate against COVID-19: Results from a phase 1, dose-escalation study
- PMID: 39422261
- PMCID: PMC11492660
- DOI: 10.1080/21645515.2024.2408863
Safety and immunogenicity of a modified mRNA-lipid nanoparticle vaccine candidate against COVID-19: Results from a phase 1, dose-escalation study
Abstract
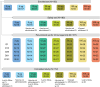
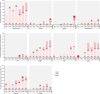
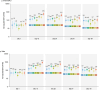
This phase 1, open-label, dose-escalation, multi-center study (NCT05477186) assessed the safety and immunogenicity of a booster dose of an mRNA COVID-19 vaccine (CV0501) encoding the SARS-CoV-2 Omicron BA.1 spike protein. Participants aged ≥ 18 years previously vaccinated with ≥ 2 doses of an mRNA COVID-19 vaccine received CV0501 doses ranging from 12 to 200 μg. After assessment of safety and immunogenicity of the 12 μg dose in 30 adults, 30 adults ≤ 64 years were randomized to receive either a 3 or 6 μg dose. Solicited adverse events (AEs) were collected for 7 days, unsolicited AEs for 28 days, and serious AEs (SAEs), medically attended AEs (MAAEs), and AEs of special interest (AESIs) until day (D) 181 post-vaccination. Serum neutralizing titers specific to SARS-CoV-2 BA.1, wild-type, Delta, and additional Omicron subvariants were assessed at D1, D15, D29, D91, and D181. Of 180 vaccinated participants (mean age: 49.3 years; 57.8% women), 70.6% had prior SARS-CoV-2 infection. Most solicited local (98.1%) and systemic (96.7%) AEs were of mild-to-moderate severity; the most common were injection site pain (57.5%; 33.3-73.3% across groups) and myalgia (36.9%; 13.3-56.7%). Unsolicited AEs were reported by 14.4% (6.7-26.7%) of participants (mild-to-moderate severity in 88.5% of the participants). Three participants (1.7%) reported SAEs, 16.7% (6.7-30.0%) reported MAAEs, and 8.3% (0.0-13.3%) reported AESIs (15 COVID-19 cases), none related to vaccination. Geometric means of serum neutralizing titers increased from baseline to D15 and D29 (dose-dependent), and then decreased over time. The safety and immunogenicity results supported advancement to a phase 2 trial.
Keywords: COVID-19; SARS-CoV-2 variants; booster; clinical trial; immunogenicity; mRNA vaccine; safety.
Plain language summary
What is the context? Since 2019, more than 776 million people have been infected with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) worldwide, and more than 7 million people have died due to COVID-19.The virus changes over time and new variants may evade the protection provided by vaccines that are effective against previous variants.Therefore, it is necessary to develop vaccines that can be quickly updated to better protect against COVID-19 caused by new SARS-CoV-2 variants.We developed an mRNA vaccine, CV0501, that encodes a key protein on the surface of the SARS-CoV-2 Omicron BA.1 variant to instruct the immune system for future protection against COVID-19. The mRNA is encased in lipid nanoparticles that can increase the immune response to the vaccine.What is new? We administered different dose levels (that is, different amounts of mRNA) of CV0501 to adults who had previously been vaccinated at least twice with a different COVID-19 vaccine.We found that even at increased dose levels, CV0501 caused mostly mild side effects that resolved within a few days. Serious adverse events or events that required medical attention were not related to the vaccine.We also found that CV0501 generated immune responses not only against the Omicron BA.1 subvariant (vaccine antigen) but also against the original SARS-CoV-2 variant, the Delta variant, and other Omicron subvariants, at all dose levels tested.What is the impact? Our findings indicate that the CV0501 vaccine was well tolerated and induced immune responses against vaccine target variant as well as other SARS-CoV-2 variants.Other vaccines against COVID-19 based on the mRNA technology used for CV0501 will be further evaluated.
Conflict of interest statement
MAM, CHo, SDK, PM, OOW, and SOM are employees of CureVac. MAM, SDK, PM, and SOM hold shares in CureVac. CHo and OSK received consulting fees from CureVac. PM declares CureVac patents. SB, CSe, NW, EB, TG, PBo, RR, MG, and NV are employees of GSK. CSe, EB, PBo, MG, RR, and NV hold financial equitiesshares in GSK as part of their employee remunerations. MB reports institutional research grants from MSD, ViiV Healthcare, GSK, Gilead Sciences, AbbVie, Novavax, Sanofi, Eli Lilly, Novo Nordisk, EyeGene, Cymra Life Sciences, and personal honoraria for lectures from ViiV Healthcare and Gilead Sciences; for attending educational meetings from ViiV Healthcare and Gilead Sciences; for Advisory Board participation from ViiV Healthcare and Gilead Sciences, and Data Safety Monitoring Board participation from Cymra Life Sciences and EyeGene; and for his role in the New South Wales Ministerial HIV implementation Committee. MVF reports institutional research grants from GSK for the running of the clinical trial. SM reports consulting fees from Novartis and personal honoraria for lectures from Gilead Sciences, for support for attending meetings and/or travel from Pfizer, and for participation in an Advisory Board or Data Safety Monitoring Board from MSD. BJE, CSh, MGDI, PBr, AP, and JS have no conflicts of interest. The authors declare no other financial or non-financial relationships or activities.
Figures


References
-
- World Health Organization . WHO Coronavirus (COVID-19) Dashboard. 2024. [accessed 2024 Sep 3]. http://covid19.who.int/table.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
