Probing Phase Formation and Structural Transformations in Sodium Extraction and Insertion of NaFe1- yMnyPO4 through First-Principles Calculations
- PMID: 39422350
- PMCID: PMC11618992
- DOI: 10.1021/acs.inorgchem.4c03148
Probing Phase Formation and Structural Transformations in Sodium Extraction and Insertion of NaFe1- yMnyPO4 through First-Principles Calculations
Abstract
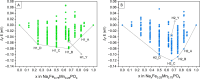
Manganese (Mn) substitution is a widely explored strategy aimed at sustainably enhancing the energy density of iron (Fe)-based electrode materials by taking advantage of the higher redox potential of the former. However, excessive Mn content can lead to detrimental effects, offsetting the expected improvements. In experimental studies, triphylite NaFe0.8Mn0.2PO4 has been identified as an optimal composition with enhanced electrochemical performance compared to that of its parent phase NaFePO4. Higher Mn contents result in a loss of capacity and increased voltage hysteresis. In this study, density functional theory (DFT) calculations were employed to investigate the phase stability upon desodiation of Mn-poor and -rich NaxFe1-yMnyPO4 compositions. Our findings reveal distinct stability behaviors in antagonistic systems NaxFe0.75Mn0.25PO4 and NaxFe0.25Mn0.75PO4, where the presence of Na-vacancies and charge orderings appear to influence phase stability. In addition, the number of intermediate phases throughout the desodiation process is identified as a crucial factor in buffering the volume changes. This work sheds light on the superior electrochemical performance of lightly Mn-substituted phases and unveils a key parameter for designing future electrode materials with improved performance.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
-
- Pan H.; Hu Y.-S.; Chen L. Room-temperature stationary sodium-ion batteries for large-scale electric energy storage. Energy Environ. Sci. 2013, 6 (8), 2338–2360. 10.1039/C3EE40847G. - DOI
-
- He L.; Li H.; Ge X.; Li S.; Wang X.; Wang S.; Zhang L.; Zhang Z. Iron-phosphate-based cathode materials for cost-effective sodium-ion batteries: development, challenges, and prospects. Adv. Mater. Interfaces 2022, 9 (20), 220051510.1002/admi.202200515. - DOI
-
- Hirsh H. S.; Li Y.; Tan D. H.; Zhang M.; Zhao E.; Meng Y. S. Sodium-ion batteries paving the way for grid energy storage. Adv. Energy Mater. 2020, 10 (32), 200127410.1002/aenm.202001274. - DOI
-
- Kim S. W.; Seo D. H.; Ma X.; Ceder G.; Kang K. Electrode materials for rechargeable sodium-ion batteries: potential alternatives to current lithium-ion batteries. Adv. Energy Mater. 2012, 2 (7), 710–721. 10.1002/aenm.201200026. - DOI
LinkOut - more resources
Full Text Sources

