Linezolid Pharmacokinetic-Anemia Modeling in Children With Rifampicin-Resistant Tuberculosis
- PMID: 39422476
- PMCID: PMC11650862
- DOI: 10.1093/cid/ciae497
Linezolid Pharmacokinetic-Anemia Modeling in Children With Rifampicin-Resistant Tuberculosis
Abstract
Background: Linezolid, a component of rifampicin-resistant/multidrug-resistant tuberculosis (RR/MDR-TB) treatment, is associated with treatment-limiting toxicities, including anemia. Patient-level and linezolid pharmacokinetic risk factors for anemia have not been well described in children treated for RR/MDR-TB.
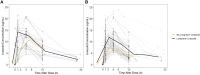
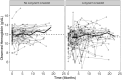
Methods: We evaluated the pharmacokinetics of linezolid and longitudinal hemoglobin data to validate an existing population linezolid pharmacokinetic model. We assessed the impact of linezolid pharmacokinetics and the risk of developing anemia in a prospectively enrolled cohort of children. A previously published population pharmacokinetic linezolid model was validated using nonlinear mixed effects modeling. A multivariable ordinal logistic regression model was built to predict the incidence of anemia.
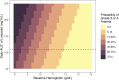
Results: A total of 112 children, median age 7.2 years (interquartile range, 2.2-16.3), were included from South Africa (n = 87) and India (n = 25). Of these, 24 children contributed new linezolid pharmacokinetic data. The population pharmacokinetic model, which informs the currently recommended linezolid dosing in children (10-15 mg/kg), was validated with these additional new data. For every 1 g/dL lower baseline hemoglobin level, the odds of developing grade 3 or 4 anemia increased by 2.64 (95% confidence interval [CI], 1.98-3.62). For every 1 mg/L × h higher linezolid area under the concentration-time curve, the odds of developing grade 3 or 4 anemia increased by 1.012 (95% CI, 1.007-1.017).
Conclusions: Taken together, these data confirm currently recommended linezolid doses for children. The risk of anemia in children should be carefully considered and monitored. Initiating linezolid in children with low baseline hemoglobin increases the probability of experiencing grade 3 or 4 anemia.
Keywords: anemia; children; dosing; linezolid; pharmacokinetics.
© The Author(s) 2024. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest . The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
Figures


References
-
- Azzouz A, Preuss CV. Linezolid. StatPearls. Available at: https://www.ncbi.nlm.nih.gov/books/NBK53979. Accessed 3 September 2023.
-
- Sotgiu G, Centis R, D’Ambrosio L, Spanevello A, Migliori GB; International Group for the Study of Linezolid . Linezolid to treat extensively drug-resistant TB: retrospective data are confirmed by experimental evidence. Eur Respir J 2013; 42:288–90. - PubMed
-
- Cox H, Ford N, Hughes J, Goemaere E. Linezolid for multidrug-resistant tuberculosis. Lancet Infect Dis 2013; 13:16. - PubMed
-
- Garcia-Prats AJ, Rose PC, Hesseling AC, Schaaf HS. Linezolid for the treatment of drug-resistant tuberculosis in children: a review and recommendations. Tuberculosis (Edinb) 2014; 94:93–104. - PubMed

