Short-range C-signaling restricts cheating behavior during Myxococcus xanthus development
- PMID: 39422488
- PMCID: PMC11559036
- DOI: 10.1128/mbio.02440-24
Short-range C-signaling restricts cheating behavior during Myxococcus xanthus development
Abstract
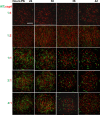
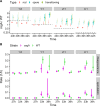
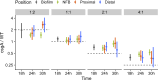
Myxococcus xanthus uses short-range C-signaling to coordinate multicellular mound formation with sporulation during fruiting body development. A csgA mutant deficient in C-signaling can cheat on wild type (WT) in mixtures and form spores disproportionately, but our understanding of cheating behavior is incomplete. We subjected mixtures of WT and csgA cells at different ratios to co-development and used confocal microscopy and image analysis to quantify the arrangement and morphology of cells. At a ratio of one WT to four csgA cells (1:4), mounds failed to form. At 1:2, only a few mounds and spores formed. At 1:1, mounds formed with a similar number and arrangement of WT and csgA rods early in development, but later the number of csgA spores near the bottom of these nascent fruiting bodies (NFBs) exceeded that of WT. This cheating after mound formation involved csgA forming spores at a greater rate, while WT disappeared at a greater rate, either lysing or exiting NFBs. At 2:1 and 4:1, csgA rods were more abundant than expected throughout the biofilm both before and during mound formation, and cheating continued after mound formation. We conclude that C-signaling restricts cheating behavior by requiring sufficient WT cells in mixtures. Excess cheaters may interfere with positive feedback loops that depend on the cellular arrangement to enhance C-signaling during mound building. Since long-range signaling could not likewise communicate the cellular arrangement, we propose that C-signaling was favored evolutionarily and that other short-range signaling mechanisms provided selective advantages in bacterial biofilm and multicellular animal development.
Importance: Bacteria communicate using both long- and short-range signals. Signaling affects community composition, structure, and function. Adherent communities called biofilms impact medicine, agriculture, industry, and the environment. To facilitate the manipulation of biofilms for societal benefits, a better understanding of short-range signaling is necessary. We investigated the susceptibility of short-range C-signaling to cheating during Myxococcus xanthus biofilm development. A mutant deficient in C-signaling fails to form mounds containing spores (i.e., fruiting bodies) but cheats on C-signaling by wild type in starved cell mixtures and forms spores disproportionately. We found that cheating requires sufficient wild-type cells in the initial mix and can occur both before mound formation and later during the sporulation stage of development. By restricting cheating behavior, short-range C-signaling may have been favored evolutionarily rather than long-range diffusible signaling. Cheating restrictions imposed by short-range signaling may have likewise driven the evolution of multicellularity broadly.
Keywords: Myxococcus xanthus; bacterial development; biofilms; cheating; evolution; extracellular signaling; fruiting body; multicellular development; short-range signaling; spores.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Cell density, alignment, and orientation correlate with C-signal-dependent gene expression during Myxococcus xanthus development.Proc Natl Acad Sci U S A. 2021 Nov 9;118(45):e2111706118. doi: 10.1073/pnas.2111706118. Proc Natl Acad Sci U S A. 2021. PMID: 34732578 Free PMC article.
-
Transcriptomic analysis of Myxococcus xanthus csgA, fruA, and mrpC mutants reveals extensive and diverse roles of key regulators in the multicellular developmental process.BMC Genomics. 2025 Apr 8;26(1):355. doi: 10.1186/s12864-025-11417-z. BMC Genomics. 2025. PMID: 40200151 Free PMC article.
-
Ultrasensitive Response of Developing Myxococcus xanthus to the Addition of Nutrient Medium Correlates with the Level of MrpC.J Bacteriol. 2018 Oct 23;200(22):e00456-18. doi: 10.1128/JB.00456-18. Print 2018 Nov 15. J Bacteriol. 2018. PMID: 30181127 Free PMC article.
-
Signaling in myxobacteria.Annu Rev Microbiol. 2004;58:75-98. doi: 10.1146/annurev.micro.58.030603.123620. Annu Rev Microbiol. 2004. PMID: 15487930 Review.
-
Two-Component Signal Transduction Systems That Regulate the Temporal and Spatial Expression of Myxococcus xanthus Sporulation Genes.J Bacteriol. 2015 Sep 14;198(3):377-85. doi: 10.1128/JB.00474-15. Print 2016 Feb 1. J Bacteriol. 2015. PMID: 26369581 Free PMC article. Review.
Cited by
-
Chimeric aggregative multicellularity in absence of kin discrimination.bioRxiv [Preprint]. 2024 Dec 4:2024.12.04.626738. doi: 10.1101/2024.12.04.626738. bioRxiv. 2024. PMID: 39677713 Free PMC article. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
