Development of a new Acinetobacter baumannii pneumonia rabbit model for the preclinical evaluation of future anti-infective strategies
- PMID: 39422502
- PMCID: PMC11619384
- DOI: 10.1128/spectrum.01570-24
Development of a new Acinetobacter baumannii pneumonia rabbit model for the preclinical evaluation of future anti-infective strategies
Abstract
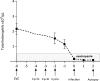
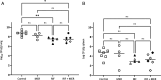
Carbapenem-resistant Acinetobacter baumannii (CRAB) is an emerging cause of hospital-acquired pneumonia (HAP). Preclinical large models are warranted to predict the efficacy and the resistance profile of anti-infectives and mimic how they will be used in the human treatment of CRAB-HAP. Here we reported on the development of an Acinetobacter baumannii experimental pneumonia model in immunocompromised rabbits, receiving a 48-h human-simulated regimen. The efficacy of meropenem (2 g/q8h i.v. over prolonged 3-h perfusion), rifampin (25 mg/kg/q8h, i.v.), or the combination of meropenem and rifampin was assessed in rabbits infected with the carbapenem-susceptible ATCC 17978 reference strain or the CRAB Turc 2 clinical strain. The emergence of rifampin mutants was also investigated. Meropenem demonstrated a strong pulmonary bacterial reduction in animals infected with the ATCC 17978 strain (unlike the CRAB strain). The high rifampin dosage was associated with a 1.3 Log10 bacterial killing on average but induced the emergence of high-level resistant mutants in 80%-100% of animals, depending on the strain. The adjunction of rifampin to meropenem did not improve the bioburden in the lungs but partially reduced the number of animals exhibiting resistant mutants, whatever the tested strain. However, this adjunctive treatment was insufficient to overcome the emergence of resistance since mutation prevention concentration-related pharmacodynamic indices were unattainable at this dose. This CRAB pneumonia rabbit model represents an innovative tool to evaluate the efficacy of new or existing therapies and will provide informative data on how they can meet the resistance pharmacodynamic targets, which now need to be investigated before deciding on clinical therapeutic regimens.IMPORTANCEWithin intensive care unit settings, carbapenem-resistant Acinetobacter baumannii (CRAB) has emerged as a frequent cause of hospital-acquired pneumonia (HAP) with poor clinical outcomes. This multidrug-resistant pathogen remains very challenging to study in clinical trials, and the U.S. Food and Drug Administration highlighted the limitations of existing small animal models for evaluating antibacterial or prophylactic strategies against such critical infections. These limitations include the difficulty in anticipating the risk of the emergence of resistance during treatment. Here we developed a new Acinetobacter baumannii pneumonia rabbit model using high inoculum. We demonstrated the emergence of resistance with rifampin, an existing antibiotic debated as a rescuing option to treat CRAB infections; and even intensified rifampin doses failed to close the mutant selection window. This CRAB pneumonia rabbit model represents a valuable tool to evaluate the efficacy of new or existing therapies and provides supportive data in antimicrobial resistance pharmacodynamics.
Keywords: Acinetobacter baumannii; innovative model; nosocomial pneumonia; pharmacodynamics; rabbit; resistance.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Garnacho-Montero J, Ortiz-Leyba C, Fernández-Hinojosa E, Aldabó-Pallás T, Cayuela A, Marquez-Vácaro JA, Garcia-Curiel A, Jiménez-Jiménez FJ. 2005. Acinetobacter baumannii ventilator-associated pneumonia: epidemiological and clinical findings. Intensive Care Med 31:649–655. doi:10.1007/s00134-005-2598-0 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

