Efficacy of ursodeoxycholic acid for bile reflux after distal gastrectomy in patients with gastric cancer: a secondary analysis of the PEGASUS-D randomized clinical trial
- PMID: 39422533
- PMCID: PMC11634197
- DOI: 10.1097/JS9.0000000000002127
Efficacy of ursodeoxycholic acid for bile reflux after distal gastrectomy in patients with gastric cancer: a secondary analysis of the PEGASUS-D randomized clinical trial
Abstract
Background: Few studies have been conducted on the prevention of bile reflux in gastric cancer patients who have undergone gastrectomy. The aim of this study was to evaluate the efficacy and safety of ursodeoxycholic acid (UDCA) in preventing bile reflux after gastrectomy in patients with gastric cancer.
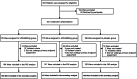
Methods: This study was a secondary analysis of the PEGASUS-D trial, a randomized, double-blind, placebo-controlled clinical trial. Adults with a diagnosis of gastric cancer who underwent gastrectomy were enrolled. Eligible participants were randomly assigned to receive 300 mg of UDCA, 600 mg of UDCA, or placebo at a ratio of 1:1:1. UDCA and placebo were administered daily for 52 weeks. The primary outcomes included bile reflux symptoms at each time point, the percentage of participants with bile reflux, and the grade of gastritis.
Results: Among 521 participants who underwent randomization, 151, 164, and 150 participants were analyzed from the 300 mg UDCA, 600 mg UDCA, and placebo groups, respectively. The difference in symptoms between the three groups was not significant. Bile reflux was less evident in the UDCA group than in the placebo group; however, this difference was significant only in the 300 mg group at 12 months postoperation (odds ratio, 0.44; P =0.0076). A significant reduction in gastritis was also observed in the 300 mg group at 12 months postoperation (odds ratio, 0.50; P =0.0368) compared to the placebo group.
Conclusions: This study showed that UDCA administration significantly reduced bile reflux and gastritis by ~50% at the 12 months-postoperative follow-up in patients who underwent gastrectomy for gastric cancer.
Copyright © 2024 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
Dr S.H. Lee reported receiving speaker honoraria and grants from Daewoong Pharmaceutical Co Ltd during the conduct of the study. Dr Yoo reported receiving grants from Daewoong Pharmaceutical Co Ltd during the conduct of the study. Dr Y. S. Park reported receiving grants from Daewoong Pharmaceutical Co Ltd during the conduct of the study. Dr D. J. Park reported receiving speaker honoraria from Daewoong Pharmaceutical Co Ltd during the conduct of the study; and a research grant from Medtronic outside the submitted work. No other disclosures were reported.
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Figures
References
-
- Miwa K, Hattori T, Miyazaki I. Duodenogastric reflux and foregut carcinogenesis. Cancer 1995;75(6 Suppl):1426–1432. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical