Enzalutamide versus abiraterone acetate in the development of new-onset or worsening type 2 diabetes mellitus in patients with metastatic castration-resistant prostate cancer: EVADE study
- PMID: 39422767
- PMCID: PMC11489205
- DOI: 10.1007/s00345-024-05280-y
Enzalutamide versus abiraterone acetate in the development of new-onset or worsening type 2 diabetes mellitus in patients with metastatic castration-resistant prostate cancer: EVADE study
Abstract
Purpose: To determine new-onset or worsening T2DM risk in patients with metastatic castration-resistant prostate cancer (mCRPC) receiving abiraterone acetate (AA) vs. enzalutamide (ENZA) in England.
Methods: Records of patients on AA and/or ENZA (2015-2021) were analysed retrospectively from UK- or England-wide databases and data sets. The primary endpoint was new-onset or worsening T2DM, analysed using a Cox model.
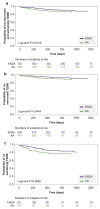
Results: Of 1382 patients, 84 (6.1%) met the primary endpoint; 42 of 826 patients (5.1%) received ENZA and 42 of 556 patients (7.6%) received AA. Among patients without baseline T2DM (n = 1049), 50 developed new-onset T2DM: 24 (3.9%) on ENZA and 26 (5.9%) on AA. Among patients with baseline T2DM (n = 333), 34 (10.2%) had worsening T2DM: 18 (8.3%) on ENZA and 16 (13.8%) on AA. Patients on ENZA had longer median follow-up (445 vs. 408 days) and treatment duration (164 vs. 139 days) than those on AA, who were also more likely to have new-onset or worsening T2DM than those on ENZA (HR: 1.8; 95% CI: 1.4-2.7; P = 0.0101). The number needed to harm for an additional patient to experience new-onset or worsening T2DM when receiving AA instead of ENZA was 40 overall, 50 in patients without baseline T2DM, and 18 in patients with baseline T2DM.
Conclusion: Patients with mCRPC receiving AA were more likely to experience new-onset or worsening T2DM than those on ENZA, despite having a shorter treatment duration. Further research is required to substantiate these findings in earlier disease settings with longer treatment duration.
Keywords: Abiraterone; Androgen receptor pathway inhibitor; Enzalutamide; Metastatic castration-resistant prostate cancer; Type 2 diabetes mellitus.
© 2024. The Author(s).
Conflict of interest statement
AB received support for the present publication from Astellas; received payment or honoraria for lectures, presentations, speakers bureaus, publication writing, or educational events from Astellas and Janssen; received research funding to their institution and support for attending meetings and/or travel from Janssen; and participated on an advisory board for Janssen. AC, AP, NR, RS, and KM are full-time employees of Astellas Pharma Europe Ltd., Surrey, UK. HS was a full-time employee of Astellas Pharma Europe Ltd., Surrey, UK, at the time of the study. DC received support for the present publication from Astellas. AM received support for the present publication from Astellas; received grants or contracts, consulting fees, payment, or honoraria for lectures, presentations, speakers bureaus, publication writing, or educational events, as well as support for attending meetings and/or travel from AstraZeneca, Astellas, Bayer, Bristol-Myers Squibb, Ferring, Ipsen, Janssen, EUSAPharm, MSD, Merck Serono, Novartis, Takeda, Teva, Pfizer, Recordati, and Roche; and holds leadership or fiduciary roles in the EAU and DGU boards of directors.
Figures

Comment in
-
From testosterone to mitochondria: unveiling the metabolic consequences of abiraterone therapy.World J Urol. 2025 Feb 10;43(1):112. doi: 10.1007/s00345-025-05457-z. World J Urol. 2025. PMID: 39928129 No abstract available.
References
-
- Sung H, Ferlay J, Siegel RL et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71:209–249. 10.3322/caac.21660 - PubMed
-
- Cancer Research UK Prostate cancer statistics. https://www.cancerresearchuk.org/health-professional/cancer-statistics/s.... Accessed 18 December 2023
-
- Cancer Research UK Survival of prostate cancer. https://www.cancerresearchuk.org/about-cancer/prostate-cancer/survival. Accessed 18 December 2023
-
- Yang X, Chen H, Xu D et al (2022) Efficacy and safety of Androgen Deprivation Therapy (ADT) combined with modified docetaxel chemotherapy versus ADT combined with standard docetaxel chemotherapy in patients with metastatic castration-resistant prostate cancer: study protocol for a multicentre prospective randomized controlled trial. BMC Cancer 22:177. 10.1186/s12885-022-09276-y - PMC - PubMed
-
- National Institute for Health and Care Excellence Abiraterone for treating metastatic hormone-relapsed prostate cancer before chemotherapy is indicated. Technology appraisal guidance [TA387]. https://www.nice.org.uk/guidance/ta387/chapter/1-Recommendations. Accessed 18 December 2023
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

