A new gene signature for endothelial senescence identifies self-RNA sensing by retinoic acid-inducible gene I as a molecular facilitator of vascular aging
- PMID: 39422883
- PMCID: PMC11488300
- DOI: 10.1111/acel.14240
A new gene signature for endothelial senescence identifies self-RNA sensing by retinoic acid-inducible gene I as a molecular facilitator of vascular aging
Abstract
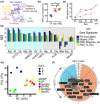
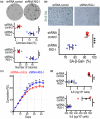
The number of senescent vascular endothelial cells increases during aging and their dysfunctional phenotype contributes to age-related cardiovascular disease. Identification of senescent cells is challenging as molecular changes are often tissue specific and occur amongst clusters of normal cells. Here, we established, benchmarked, and validated a new gene signature called EndoSEN that pinpoints senescent endothelial cells. The EndoSEN signature was enriched for interferon-stimulated genes (ISG) and correlated with the senescence-associated secretory phenotype (SASP). SASP establishment is classically attributed to DNA damage and cyclic GMP-AMP synthase activation, but our results revealed a pivotal role for RNA accumulation and sensing in senescent endothelial cells. Mechanistically, we showed that endothelial cell senescence hallmarks include self-RNA accumulation, RNA sensor RIG-I upregulation, and an ISG signature. Moreover, a virtual model of RIG-I knockout in endothelial cells underscored senescence as a key pathway regulated by this sensor. We tested and confirmed that RIG-I knockdown was sufficient to extend the lifespan and decrease the SASP in endothelial cells. Taken together, our evidence suggests that targeting RNA sensing is a potential strategy to delay vascular aging.
Keywords: RNA sensing; cardiovascular diseases; cellular senescence; endothelial cells; senescence‐associated secretory phenotype.
© 2024 The Author(s). Aging Cell published by Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures




References
-
- Andersen, J. B. , Li, X. L. , Judge, C. S. , Zhou, A. , Jha, B. K. , Shelby, S. , Zhou, L. , Silverman, R. H. , & Hassel, B. A. (2007). Role of 2‐5A‐dependent RNase‐L in senescence and longevity. Oncogene, 26, 3081–3088. - PubMed
-
- Bochenek, M. L. , Schütz, E. , & Schäfer, K. (2016). Endothelial cell senescence and thrombosis: ageing clots. Thrombosis Research, 147, 36–45. - PubMed
-
- Chan, M. , Yuan, H. , Soifer, I. , Maile, T. M. , Wang, R. Y. , Ireland, A. , O'Brien, J. , Goudeau, J. , Chan, L. , Vijay, T. , Freund, A. , Kenyon, C. , Bennett, B. , McAllister, F. , Kelley, D. R. , Roy, M. , Cohen, R. L. , Levinson, A. D. , Botstein, D. , & Hendrickson, D. G. (2022). Novel insights from a multiomics dissection of the Hayflick limit. eLife, 11, e70283. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

