Time to Sustained Recovery Among Outpatients With COVID-19 Receiving Montelukast vs Placebo: The ACTIV-6 Randomized Clinical Trial
- PMID: 39422912
- PMCID: PMC11581631
- DOI: 10.1001/jamanetworkopen.2024.39332
Time to Sustained Recovery Among Outpatients With COVID-19 Receiving Montelukast vs Placebo: The ACTIV-6 Randomized Clinical Trial
Abstract
Importance: The effect of montelukast in reducing symptom duration among outpatients with mild to moderate COVID-19 is uncertain.
Objective: To assess the effectiveness of montelukast compared with placebo in treating outpatients with mild to moderate COVID-19.
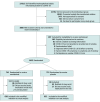
Design, setting, and participants: This randomized clinical trial (Accelerating COVID-19 Therapeutic Interventions and Vaccines [ACTIV]-6) was conducted from January 27 through June 23, 2023, during the circulation of Omicron subvariants. Participants aged 30 years or older with confirmed SARS-CoV-2 infection and 2 or more acute COVID-19 symptoms for less than 7 days were included across 104 US sites.
Interventions: Participants were randomized 1:1 to receive montelukast, 10 mg once daily, or matched placebo for 14 days.
Main outcomes and measures: The primary outcome was time to sustained recovery (defined as ≥3 consecutive days without symptoms). Secondary outcomes included time to death; time to hospitalization or death; a composite of health care utilization events (hospitalization, urgent care clinic visit, emergency department visit, or death); COVID-19 clinical progression scale score; and difference in mean time unwell. A modified intention-to-treat approach was used for the analysis.
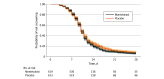
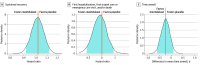
Results: Among 1250 participants who were randomized and received the study drug or placebo, the median age was 53 years (IQR, 42-62 years), 753 (60.2%) were female, and 704 (56.3%) reported receiving 2 or more doses of a SARS-CoV-2 vaccine. Among 628 participants who received montelukast and 622 who received placebo, differences in time to sustained recovery were not observed (adjusted hazard ratio [AHR], 1.02; 95% credible interval [CrI], 0.92-1.12; P = .63 for efficacy). Unadjusted median time to sustained recovery was 10 days (95% CI, 10-11 days) in both groups. No deaths occurred, and hospitalizations were reported for 2 participants (0.3%) in each group; the composite of health care utilization events was reported for 18 participants (2.9%) in the montelukast group and 18 (2.9%) in the placebo group (AHR, 1.01; 95% CrI, 0.45-1.84; P = .48 for efficacy). Five participants (0.4%) experienced serious adverse events (3 [0.5%] in the montelukast group and 2 [0.3%] in the placebo group).
Conclusions and relevance: In this randomized clinical trial of outpatients with mild to moderate COVID-19, treatment with montelukast did not reduce duration of COVID-19 symptoms. These findings do not support the use of montelukast for the treatment of mild to moderate COVID-19.
Trial registration: ClinicalTrials.gov Identifier: NCT04885530.
Conflict of interest statement
Figures
Comment in
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

