Bioenergetic-active exosomes for cartilage regeneration and homeostasis maintenance
- PMID: 39423269
- PMCID: PMC11488572
- DOI: 10.1126/sciadv.adp7872
Bioenergetic-active exosomes for cartilage regeneration and homeostasis maintenance
Abstract
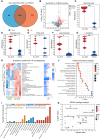
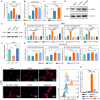
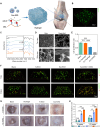
Cartilage regeneration relies on adequate and continuous bioenergy supply to facilitate cellular differentiation and extracellular matrix synthesis. Chondrocytes frequently undergo energy stress under pathological conditions, characterized by disrupted cellular metabolism and reduced adenosine triphosphate (ATP) levels. However, there has limited progress in modulating energy metabolism for cartilage regeneration thus far. Here, we developed bioenergetic-active exosomes (Suc-EXO) to promote cartilage regeneration and homeostasis maintenance. Suc-EXO exhibited a 5.42-fold increase in ATP content, enabling the manipulation of cellular energy metabolism by fueling the TCA cycle. With continuous energy supply, Suc-EXO promoted BMSC chondrogenic differentiation via the P2X7-mediated PI3K-AKT pathway. Moreover, Suc-EXO improved chondrocyte anabolism and mitochondrial homeostasis via the P2X7-mediated SIRT3 pathway. In a rabbit cartilage defect model, the Suc-EXO-encapsulated hydrogel notably promoted cartilage regeneration and maintained neocartilage homeostasis, leading to 2.26 and 1.53 times increase in Col2 and ACAN abundance, respectively. These findings make a remarkable breakthrough in modulating energy metabolism for cartilage regeneration, offering immense potential for clinical translation.
Figures




References
-
- Marshall M., Watt F. E., Vincent T. L., Dziedzic K., Hand osteoarthritis: Clinical phenotypes, molecular mechanisms and disease management. Nat. Rev. Rheumatol. 14, 641–656 (2018). - PubMed
-
- Hunter D. J., Bierma-Zeinstra S., Osteoarthritis. Lancet 393, 1745–1759 (2019). - PubMed
-
- Sharma L., Osteoarthritis of the knee. N. Engl. J. Med. 384, 51–59 (2021). - PubMed
-
- Bijlsma J. W., Berenbaum F., Lafeber F. P., Osteoarthritis: An update with relevance for clinical practice. Lancet 377, 2115–2126 (2011). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

