Lineage tracing of stem cell-derived dopamine grafts in a Parkinson's model reveals shared origin of all graft-derived cells
- PMID: 39423273
- PMCID: PMC11488568
- DOI: 10.1126/sciadv.adn3057
Lineage tracing of stem cell-derived dopamine grafts in a Parkinson's model reveals shared origin of all graft-derived cells
Abstract
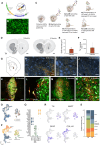
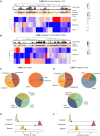
Stem cell therapies for Parkinson's disease are at an exciting time of development, and several clinical trials have recently been initiated. Human pluripotent stem cells are differentiated into transplantable dopamine (DA) progenitors which are proliferative at the time of grafting and undergo terminal differentiation and maturation in vivo. While the progenitors are homogeneous at the time of transplantation, they give rise to heterogeneous grafts composed not only of therapeutic DA neurons but also of other mature cell types. The mechanisms for graft diversification are unclear. We used single-nucleus RNA-seq and ATAC-seq to profile DA progenitors before transplantation combined with molecular barcode-based tracing to determine origin and shared lineages of the mature cell types in the grafts. Our data demonstrate that astrocytes, vascular leptomeningeal cells, and DA neurons are the main component of the DAergic grafts, originating from a common progenitor that is tripotent at the time of transplantation.
Figures



References
-
- Barker R. A., Drouin-Ouellet J., Parmar M., Cell-based therapies for Parkinson disease—Past insights and future potential. Nat. Rev. Neurol. 11, 492–503 (2015). - PubMed
-
- Kirkeby A., Nelander J., Hoban D. B., Rogelius N., Bjartmarz H., Storm P., Fiorenzano A., Adler A. F., Vale S., Mudannayake J., Zhang Y., Cardoso T., Mattsson B., Landau A. M., Glud A. N., Sørensen J. C., Lillethorup T. P., Lowdell M., Carvalho C., Bain O., van Vliet T., Lindvall O., Björklund A., Harry B., Cutting E., Widner H., Paul G., Barker R. A., Parmar M., Preclinical quality, safety, and efficacy of a human embryonic stem cell-derived product for the treatment of Parkinson’s disease, STEM-PD. Cell Stem Cell 30, 1299–1314 (2023). - PubMed
-
- Piao J., Zabierowski S., Dubose B. N., Hill E. J., Navare M., Claros N., Rosen S., Ramnarine K., Horn C., Fredrickson C., Wong K., Safford B., Kriks S., El Maarouf A., Rutishauser U., Henchcliffe C., Wang Y., Riviere I., Mann S., Bermudez V., Irion S., Studer L., Tomishima M., Tabar V., Preclinical efficacy and safety of a human embryonic stem cell-derived midbrain dopamine progenitor product, MSK-DA01. Cell Stem Cell 28, 217–229.e7 (2021). - PMC - PubMed
-
- Tiklová K., Nolbrant S., Fiorenzano A., Björklund Å. K., Sharma Y., Heuer A., Gillberg L., Hoban D. B., Cardoso T., Adler A. F., Birtele M., Lundén-Miguel H., Volakakis N., Kirkeby A., Perlmann T., Parmar M., Single cell transcriptomics identifies stem cell-derived graft composition in a model of Parkinson’s disease. Nat. Commun. 11, 2434 (2020). - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

