Delayed-and-abbreviated environmental enrichment after traumatic brain injury confers neurobehavioral benefits similar to immediate-and-continuous exposure
- PMID: 39423964
- PMCID: PMC12107718
- DOI: 10.1016/j.brainres.2024.149281
Delayed-and-abbreviated environmental enrichment after traumatic brain injury confers neurobehavioral benefits similar to immediate-and-continuous exposure
Abstract
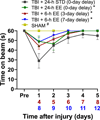
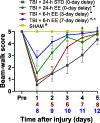
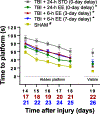
Environmental enrichment (EE) consists of increased living space, complex stimuli, and social interaction that collectively confer neurobehavioral benefits in preclinical models of traumatic brain injury (TBI). The typical EE approach entails implementation immediately after surgery and continual exposure, which is not clinically applicable, as TBI patients often only receive rehabilitation after critical care, and then only for a few hours per day. We are focused on developing a clinically relevant model of neurorehabilitation by refining the timing of initiation and duration of EE exposure after TBI. The goal of this experiment is to compare the typical EE approach to paradigms where EE is delayed by 3 or 7 days after TBI and then provided for only 6 h per day, which better mimics the clinic. The hypothesis is that the delayed-and-abbreviated EE paradigms will promote neurobehavioral benefits like the typical approach of immediate-and-continuous exposure. To test the hypothesis, anesthetized adult male rats underwent a controlled cortical impact of moderate severity (2.8 mm deformation at 4 m/s) or sham injury and then were randomly assigned to post-operative EE or standard (STD) housing. Motor ability, spatial learning, and memory retention were assessed. The hypothesis was confirmed as all EE-treated groups performed better than the STD group in all behavioral assessments (p < 0.05) and did not differ from one another (p > 0.05). The ability of EE to provide significant behavioral benefits even when delayed and delivered in moderation affords further support for EE as a preclinical model of neurorehabilitation and offers greater insight into the length of the therapeutic window.
Keywords: Behavior; Controlled cortical impact; Environmental enrichment; Learning and memory; Morris water maze; Recovery; Traumatic brain injury.
Copyright © 2024 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




References
-
- Andelic N, Bautz-Holter E, Ronning P, Olafsen K, Sigurdardottir S, Schanke AK, Sveen U, Tornas S, Sandhaug M, Roe C, 2012. Does an early onset and continuous chain of rehabilitation improve the long-term functional outcome of patients with severe traumatic brain injury? J. Neurotrauma. 29, 66–74. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

