AMH regulates a mosaic population of AMHR2-positive cells in the ovarian surface epithelium
- PMID: 39424141
- PMCID: PMC11602974
- DOI: 10.1016/j.jbc.2024.107897
AMH regulates a mosaic population of AMHR2-positive cells in the ovarian surface epithelium
Abstract
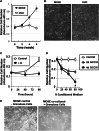
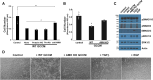
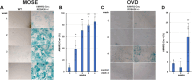
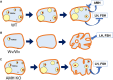
The function and homeostasis of the mammalian ovary depend on complex paracrine interactions between multiple cell types. Using primary mouse tissues and isolated cells, we showed in vitro that ovarian follicles secrete factor(s) that suppresses the growth of ovarian epithelial cells in culture. Most of the growth suppressive activity was accounted for by Anti-Mullerian Hormone/Mullerian Inhibitory Substance (AMH/MIS) secreted by granulosa cells of the follicles, as determined by immune depletion experiments. Additionally, conditioned medium from granulosa cells from wild-type control, but not AMH knockout, suppressed epithelial cell growth. Tracing of the AMH-regulated cells using AMHR2 (AMH receptor 2)-Cre:ROSA26 mutant mice indicated the presence of populations of AMHR2-positive epithelial cells on the ovarian surface and oviduct epithelia. Cells isolated from the mutant mice indicated that a subpopulation of cells marked by AMHR2-Cre:ROSA26 accounted for most cell growth and expansion in ovarian surface epithelial cells, and the AMHR2 lineage-derived cells were regulated by AMH in vitro; whereas, fewer AMHR2-Cre:ROSA26-marked cells accounted for oviduct epithelial cell outgrowth. The results reveal a paracrine pathway in maintaining follicle-epithelial homeostasis in the ovary and support a subpopulation of AMHR2 lineage marked epithelial cells as ovarian epithelial stem/progenitor cells with higher proliferative potential regulatable by follicle-secreted AMH.
Keywords: AMHR2; MIS/AMH; granulosa cell; ovarian cancer; ovarian epithelium; oviduct epithelium; stem cell.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures




References
-
- Lorenzo H.K., Teixeira J., Pahlavan N., Laurich V.M., Donahoe P.K., MacLaughlin D.T. New approaches for high-yield purification of Müllerian inhibiting substance improve its bioactivity. J. Chromatogr. B Analyt Technol. Biomed. Life Sci. 2002;766:89–98. - PubMed
-
- Eppig J.J. Reproduction: oocytes call, granulosa cells connect. Curr. Biol. 2018;28:R354–R356. - PubMed
-
- Schulz B.O., Krebs D., Diedrich K., Knoll H., Hobbel K., Hamerich U. Effects of granulosa cells and gonadotrophins on maturation of rabbit oocytes in vitro. Arch. Gynecol. 1985;236:135–143. - PubMed
-
- Matzuk M.M., Burns K.H., Viveiros M.M., Eppig J.J. Intercellular communication in the mammalian ovary: oocytes carry the conversation. Science. 2002;296:2178–2180. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

