Neural Correlates of Category Learning in Monkey Inferior Temporal Cortex
- PMID: 39424365
- PMCID: PMC11622174
- DOI: 10.1523/JNEUROSCI.0312-24.2024
Neural Correlates of Category Learning in Monkey Inferior Temporal Cortex
Abstract
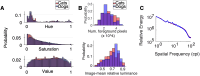
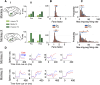
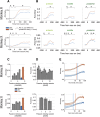
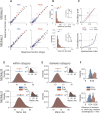
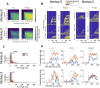
Area TE is required for normal learning of visual categories based on perceptual similarity. To evaluate whether category learning changes neural activity in area TE, we trained two monkeys (both male) implanted with multielectrode arrays to categorize natural images of cats and dogs. Neural activity during a passive viewing task was compared pre- and post-training. After the category training, the accuracy of abstract category decoding improved. Single units became more category selective, the proportion of single units with category selectivity increased, and units sustained their category-specific responses for longer. Visual category learning thus appears to enhance category separability in area TE by driving changes in the stimulus selectivity of individual neurons and by recruiting more units to the active network.
Keywords: area TE; categorization; electrophysiology; inferotemporal cortex; monkey; vision.
Copyright © 2024 the authors.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


Update of
-
Neural correlates of category learning in monkey inferior temporal cortex.bioRxiv [Preprint]. 2023 Dec 7:2023.12.05.568765. doi: 10.1101/2023.12.05.568765. bioRxiv. 2023. Update in: J Neurosci. 2024 Dec 4;44(49):e0312242024. doi: 10.1523/JNEUROSCI.0312-24.2024. PMID: 38168336 Free PMC article. Updated. Preprint.
References
-
- Anon (n.d.) Web content accessibility guidelines (WCAG) 2.0. Available at: https://www.w3.org/TR/WCAG20/. Accessed October 30, 2023.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
