Metabotropic NMDAR Signaling Contributes to Sex Differences in Synaptic Plasticity and Episodic Memory
- PMID: 39424366
- PMCID: PMC11638816
- DOI: 10.1523/JNEUROSCI.0438-24.2024
Metabotropic NMDAR Signaling Contributes to Sex Differences in Synaptic Plasticity and Episodic Memory
Abstract
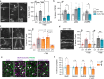
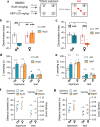
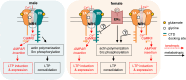
NMDA receptor (NMDAR)-mediated calcium influx triggers the induction and initial expression of long-term potentiation (LTP). Here we report that in male rodents, ion flux-independent (metabotropic) NMDAR signaling is critical for a third step in the production of enduring LTP, i.e., cytoskeletal changes that stabilize the activity-induced synaptic modifications. Surprisingly, females rely upon estrogen receptor alpha (ERα) for the metabotropic NMDAR operations used by males. Blocking NMDAR channels with MK-801 eliminated LTP expression in hippocampal field CA1 of both sexes but left intact theta burst stimulation (TBS)-induced actin polymerization within dendritic spines. A selective antagonist (Ro25-6981) of the NMDAR GluN2B subunit had minimal effects on synaptic responses but blocked actin polymerization and LTP consolidation in males only. Conversely, an ERα antagonist thoroughly disrupted TBS-induced actin polymerization and LTP in females while having no evident effect in males. In an episodic memory paradigm, Ro25-6981 prevented acquisition of spatial locations by males but not females, whereas an ERα antagonist blocked acquisition in females but not males. Sex differences in LTP consolidation were accompanied by pronounced differences in episodic memory in tasks involving minimal (for learning) cue sampling. Males did better on acquisition of spatial information whereas females had much higher scores than males on tests for acquisition of the identity of cues (episodic "what") and the order in which the cues were sampled (episodic "when"). We propose that sex differences in synaptic processes used to stabilize LTP result in differential encoding of the basic elements of episodic memory.
Keywords: NMDAR; actin; estrogen receptor; hippocampus; memory; metabotropic.
Copyright © 2024 the authors.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



Update of
-
Metabotropic NMDA Receptor Signaling Contributes to Sex Differences in Synaptic Plasticity and Episodic Memory.bioRxiv [Preprint]. 2024 Jan 27:2024.01.26.577478. doi: 10.1101/2024.01.26.577478. bioRxiv. 2024. Update in: J Neurosci. 2024 Dec 11;44(50):e0438242024. doi: 10.1523/JNEUROSCI.0438-24.2024. PMID: 38328108 Free PMC article. Updated. Preprint.
References
-
- Akashi K, Kakizaki T, Kamiya H, Fukaya M, Yamasaki M, Abe M, Natsume R, Watanabe M, Sakimura K (2009) NMDA receptor GluN2B (GluR epsilon 2/NR2B) subunit is crucial for channel function, postsynaptic macromolecular organization, and actin cytoskeleton at hippocampal CA3 synapses. J Neurosci 29:10869–10882. 10.1523/JNEUROSCI.5531-08.2009 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
