Efficacy and safety of CT-P47 versus reference tocilizumab: 32-week results of a randomised, active-controlled, double-blind, phase III study in patients with rheumatoid arthritis, including 8 weeks of switching data from reference tocilizumab to CT-P47
- PMID: 39424404
- PMCID: PMC11492937
- DOI: 10.1136/rmdopen-2024-004514
Efficacy and safety of CT-P47 versus reference tocilizumab: 32-week results of a randomised, active-controlled, double-blind, phase III study in patients with rheumatoid arthritis, including 8 weeks of switching data from reference tocilizumab to CT-P47
Abstract
Objectives: To demonstrate efficacy equivalence of CT-P47 and EU-approved reference tocilizumab (r-TCZ) in patients with rheumatoid arthritis (RA).
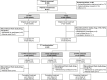
Methods: This double-blind, phase III study randomised (1:1) patients to receive CT-P47 or r-TCZ (8 mg/kg) every 4 weeks until week 20 during treatment period (TP) 1. Prior to week 24 dosing, patients receiving r-TCZ were randomised (1:1) to continue r-TCZ or switch to CT-P47; patients receiving CT-P47 continued CT-P47 (TP2, 8 mg/kg every 4 weeks until week 48). The dual primary endpoints (for different regulatory requirements) were mean changes from baseline in Disease Activity Score in 28 joints (DAS28; erythrocyte sedimentation rate (ESR)) at week 12 and week 24. Efficacy equivalence was determined if CIs for the treatment difference were within predefined equivalence margins: (95% CI -0.6, 0.6 (analysis of covariance (ANCOVA)) at week 12 or 90% CI -0.6, 0.5 (ANCOVA with multiple imputation) at week 24). Additional efficacy, pharmacokinetic (PK) and safety endpoints, including immunogenicity, were investigated. Findings up to week 32 are presented.
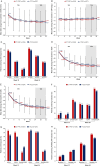
Results: In TP1, 471 patients were randomised (234 CT-P47; 237 r-TCZ). The 95% and 90% CIs for the estimated treatment differences were contained within the predefined equivalence margins; the estimated difference in DAS28-ESR at week 12 was -0.01 (95% CI -0.26, 0.24) and at week 24 was -0.10 (90% CI -0.30, 0.10). Secondary efficacy endpoints, PKs and overall safety were comparable between groups up to week 32.
Conclusions: Efficacy equivalence, alongside comparable PK, safety and immunogenicity profiles, was determined between CT-P47 and r-TCZ in adults with RA, including after switching from r-TCZ to CT-P47.
Keywords: arthritis, rheumatoid; autoimmune diseases; biosimilar pharmaceuticals.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: JSS has received payments to institution from AbbVie, AstraZeneca, Eli Lilly, Novartis, Galapagos, and Roche; personal fees from AbbVie, Amgen, Ananda, Astro, BMS, Celltrion, Inc., Chugai, Eli Lilly, Gilead, Immunovant, MSD, Novartis, Pfizer, Roche, R-Pharma, Samsung, Sanofi, and UCB; payments or honoraria from Eli Lilly; and support for meeting attendance from Eli Lilly. MK has received support for meeting attendance from Accord, Egis, Medac, and Sandoz. SJ has received consulting fees from AbbVie, Celltrion, Inc., Eli Lilly, Gilead, MSD, Novartis, Pfizer, Roche, Sanofi, and UCB; and payments or honoraria from AbbVie, Celltrion, Inc., Eli Lilly, Gilead, MSD, Novartis, Pfizer, Roche, Sanofi, and UCB. RW has received speaker fees from Eli Lilly, Janssen, Novartis, SOBI, and UCB; support for meeting attendance from AbbVie; and is involved in the leadership of the Polish Rheumatology Society. MKW has received payments or honoraria from AbbVie, Boehringer Ingelheim, Eli Lilly, Janssen, Medac, MSD, Novartis, Pfizer, Sandoz, SOBI and UCB; and support for meeting attendance from AbbVie, Medac, Novartis, Pfizer, and SOBI. PH has received research grants from Celltrion, Inc. GB has received honoraria for consulting and lectures from Celltrion, Inc., Chugai, Fresenius, and Sanofi. EK has received consulting fees/served on advisory boards for AbbVie, Celltrion, Inc., Eli Lilly, GSK, Pfizer, Sandoz, and Samsung Bioepsis; and has received speaker fees from AbbVie, Celltrion, Inc., GSK, Eli Lilly, Pfizer, and Sandoz. SHK is an employee of Celltrion, Inc. YJB, YBJ, GEY, and JWH are employees of Celltrion, Inc., and hold stocks in Celltrion, Inc. JT, AR, JJ, AZ, AD, KK, and PAK report no disclosures.
Figures

References
-
- Strand V, Khanna D. The impact of rheumatoid arthritis and treatment on patients’ lives. Clin Exp Rheumatol. 2010;28:S32–40. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
