The effect of intensive praziquantel administration on vaccine-specific responses among schoolchildren in Ugandan schistosomiasis-endemic islands (POPVAC A): an open-label, randomised controlled trial
- PMID: 39424571
- PMCID: PMC11483245
- DOI: 10.1016/S2214-109X(24)00280-8
The effect of intensive praziquantel administration on vaccine-specific responses among schoolchildren in Ugandan schistosomiasis-endemic islands (POPVAC A): an open-label, randomised controlled trial
Abstract
Background: Vaccine responses differ between populations and are often impaired in rural and low-income settings. The reasons for this are not fully understood, but observational data suggest that the immunomodulating effects of parasitic helminths might contribute. We hypothesised that Schistosoma mansoni infection suppresses responses to unrelated vaccines, and that suppression could be reversed-at least in part-by intensive praziquantel administration.
Methods: We conducted an open-label, randomised controlled trial of intensive versus standard intervention against S mansoni among schoolchildren aged 9-17 years from eight primary schools in Koome islands, Uganda. Children were randomly allocated to either an intensive group or a standard group with a computer-generated 1:1 randomisation using permuted blocks sizes 4, 6, 8, and 10. Participants in the intensive group received three praziquantel doses (approximately 40 mg/kg) 2 weeks apart before first vaccination at week 0, and every 3 months thereafter. Participants in the standard group were given one dose of approximately 40 mg/kg praziquantel after the week 8 primary endpoint. Participants in both groups received the BCG vaccine (Serum Institute of India, Pune, India) at week 0; the yellow fever (Sanofi Pasteur, Lyon, France), oral typhoid (PaxVax, London, UK), and first human papillomavirus (HPV) vaccination (Merck, Rahway, NJ, USA) at week 4; and the HPV booster and tetanus-diphtheria vaccine (Serum Institute of India) at week 28. The primary outcome was vaccine response at week 8 (except for tetanus and diphtheria, which was assessed at week 52). The primary analysis population was participants who were infected with S mansoni at baseline, determined retrospectively using either plasma circulating anodic antigen (CAA) or stool PCR. The safety population comprised all randomly allocated participants. The trial was registered at the ISRCTN Registry (ISRCTN60517191) and is complete.
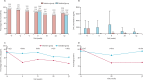
Findings: Between July 9 and Aug 14, 2019, we enrolled 478 participants, with 239 children per group. 276 (58%) participants were male and 202 (42%) participants were female. Among participants who were positive for S mansoni at baseline (171 [72%] in the intensive group and 164 [69%] in the standard group) intensive praziquantel administration significantly reduced pre-vaccination infection intensity (to median 30 CAA pg/mL [IQR 7-223] vs 1317 [243-8562], p<0·001) compared with standard treatment. Intensive praziquantel administration also reduced week 8 HPV-16-specific IgG response (geometric mean ratio 0·71 [95% CI 0·54-0·94], p=0·017), but had no effect on other primary outcomes. Among all participants (regardless of S mansoni status at baseline) intensive praziquantel administration significantly improved week 8 BCG-specific IFNγ ELISpot response (1·20 [1·01-1·43], p=0·038). Recognised adverse effects of praziquantel were reported more frequently in the intensive group. There were no recorded serious adverse events in either group.
Interpretation: We show evidence suggesting that praziquantel administration improves the BCG-specific cellular response, but not humoral responses to other vaccines. Despite observational evidence that helminths impair vaccine response, these results show minimal immediate benefits of reducing helminth burden. The effect of longer-term helminth control should be investigated.
Funding: UK Medical Research Council.
Translation: For the Luganda translation of the abstract see Supplementary Materials section.
Copyright © 2024 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests GN and AME report grants from the Wellcome Trust. GN reports funding from the EDCTP2 programme supported by the EU. AME and SC report funding from UK Medical Research Council (MRC) for conduct of the study. AME reports funding from the US National Institutes for Health, Science for Africa Foundation, the Royal Society, and DELTAS Africa, outside the submitted work. AME and AN report support from UK National Institute of Health and care Research (NIHR). AME further reports support from the Serum Institute of India, Uganda National Expanded Program on Immunization, and Emergent BioSolutions for conduct of the study. All other authors declare no competing interests.
Figures

References
-
- Black GF, Weir RE, Floyd S, et al. BCG-induced increase in interferon-gamma response to mycobacterial antigens and efficacy of BCG vaccination in Malawi and the UK: two randomised controlled studies. Lancet. 2002;359:1393–1401. - PubMed
-
- Barreto ML, Pilger D, Pereira SM, et al. Causes of variation in BCG vaccine efficacy: examining evidence from the BCG REVAC cluster randomized trial to explore the masking and the blocking hypotheses. Vaccine. 2014;32:3759–3764. - PubMed
-
- van Riet E, Adegnika AA, Retra K, et al. Cellular and humoral responses to influenza in Gabonese children living in rural and semi-urban areas. J Infect Dis. 2007;196:1671–1678. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

