The effect of intermittent preventive treatment for malaria with dihydroartemisinin-piperaquine on vaccine-specific responses among schoolchildren in rural Uganda (POPVAC B): a double-blind, randomised controlled trial
- PMID: 39424572
- PMCID: PMC11483247
- DOI: 10.1016/S2214-109X(24)00281-X
The effect of intermittent preventive treatment for malaria with dihydroartemisinin-piperaquine on vaccine-specific responses among schoolchildren in rural Uganda (POPVAC B): a double-blind, randomised controlled trial
Abstract
Background: Several important vaccines differ in immunogenicity and efficacy between populations. We hypothesised that malaria suppresses responses to unrelated vaccines and that this effect can be reversed-at least partially-by monthly malaria intermittent preventive treatment (IPT) in high-transmission settings.
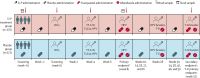
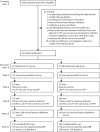
Methods: We conducted an individually randomised, double-blind, placebo-controlled trial of the effect of malaria IPT with dihydroartemisinin-piperaquine on vaccine responses among schoolchildren aged 9-17 years in Jinja district, Uganda. Participants were recruited from two schools and did not have exposure to vaccines of interest after the age of 5 years, with the exception of human papillomavirus (HPV). Computer-generated 1:1 randomisation was implemented in REDCap. 3-day courses of dihydroartemisinin-piperaquine (dosage by weight) or placebo were administered monthly, including twice before the first vaccination. Trial participants were vaccinated with the live parenteral BCG vaccine (Serum Institute of India, Pune, India) at week 0; yellow fever vaccine (YF-17D; Sanofi Pasteur, Lyon, France); live oral typhoid vaccine (Ty21a; PaxVax, London, UK), and quadrivalent virus-like particle HPV vaccine (Merck, Rahway, NJ, USA) at week 4; and toxoid vaccines (tetanus-diphtheria; Serum Institute of India) and an HPV booster at week 28. An additional HPV vaccination at week 8 was provided to female participants older than 14 years who had not previously been vaccinated, and a tetanus-diphtheria booster was given after completion of the trial at week 52. Primary outcomes were vaccine responses at week 8 and, for tetanus-diphtheria, at week 52, and analysis was done in the intention-to-treat population. Malaria parasite prevalence at enrolment and during follow-up was determined retrospectively by PCR. The safety population comprised all randomly allocated participants. The trial was registered at the ISRCTN Registry (ISRCTN62041885) and is complete.
Findings: Between May 25 and July 14, 2021, we assessed 388 potential participants for eligibility. We enrolled and randomly allocated 341 participants to the two groups (170 [50%] to dihydroartemisinin-piperaquine and 171 [50%] to placebo); 192 (56%) were female and 149 (44%) participants were male. 145 (85%) participants in the dihydroartemisinin-piperaquine group and 140 participants (82%) in the placebo group were followed up until the week 52 endpoint. At enrolment, 109 (64%) of all participants in the dihydroartemisinin-piperaquine group and 99 (58%) of 170 participants in the placebo group had malaria; this reduced to 6% or lower at all follow-up visits in the dihydroartemisinin-piperaquine group. There was no effect of dihydroartemisinin-piperaquine versus placebo on primary outcomes: BCG-specific IFNγ ELISpot response had a geometric mean ratio (GMR) of 1·09 (95% CI 0·93-1·29), p=0·28; yellow fever neutralising antibody was 1·19 (0·91-1·54), p=0·20 for plaque reduction neutralising reference tests (PRNT50) titres (the reciprocal of the last plasma dilution that reduced by 50%) and 1·24 (0·97-1·58), p=0·09 for PRNT90 titres (reciprocal of the last plasma dilution that reduced by 90%); and IgG to Salmonella enterica serovar Typhi O-lipopolysaccharide was 1·09 (0·81-1·46), p=0·58, HPV-16 was 0·72 (0·44-1·77), p=0·19, HPV-18 was 0·71 (0·47-1·09), p=0·11; tetanus toxoid was 1·22 (0·91-1·62), p=0·18, and diphtheria toxoid was 0·97 (0·83-1·13), p=0·72. There was some evidence that dihydroartemisinin-piperaquine reduced waning of the yellow fever response.
Interpretation: IPT for malaria with dihydroartemisinin-piperaquine did not improve peak vaccine responses, despite reducing malaria prevalence. Possible longer-term effects on response waning should be further explored.
Funding: UK Medical Research Council.
Translation: For the Luganda translation of the abstract see Supplementary Materials section.
Copyright © 2024 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests GN reports funding from the EDCTP2 programme supported by the EU, and from the Wellcome Trust. AME, PK and SC report funding from the UK Medical Research Council (MRC) for conduct of the study; AME reports funding from UK National Institute of Health and care Research (NIHR), Science for Africa Foundation, and DELTAS Africa, outside the submitted work. AME and SC further report support from the Serum Institute of India and Emergent BioSolutions; AME reports support from Uganda National Expanded Program on Immunization; SC reports support from Bliss GVS Pharma, India, for conduct of the study. All other authors declare no competing interests.
Figures

References
-
- WHO WHO guidelines for malaria. Oct 16, 2023. https://www.who.int/publications/i/item/guidelines-for-malaria
-
- Serazin AC, Shackelton LA, Wilson C, Bhan MK. Improving the performance of enteric vaccines in the developing world. Nat Immunol. 2010;11:769–773. - PubMed
-
- Fine PE. Variation in protection by BCG: implications of and for heterologous immunity. Lancet. 1995;346:1339–1345. - PubMed
-
- Cunnington AJ, Riley EM. Suppression of vaccine responses by malaria: insignificant or overlooked? Expert Rev Vaccines. 2010;9:409–429. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

