NLRP10 maintains epidermal homeostasis by promoting keratinocyte survival and P63-dependent differentiation and barrier function
- PMID: 39424623
- PMCID: PMC11492288
- DOI: 10.1038/s41419-024-07146-y
NLRP10 maintains epidermal homeostasis by promoting keratinocyte survival and P63-dependent differentiation and barrier function
Abstract
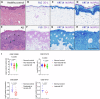
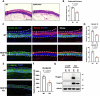
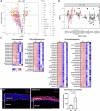
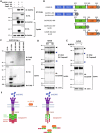
Atopic dermatitis (AD) is a common chronic inflammatory skin disorder characterized by disrupted epidermal barrier function and aberrant immune responses. Despite recent developments in new therapeutics for AD, there is still a large unmet medical need for disease management due to the complex and multifactorial nature of AD. Recent genome-wide association studies (GWAS) have identified NLRP10 as a susceptible gene for AD but the physiological role of NLRP10 in skin homeostasis and AD remains unknown. Here we show that NLRP10 is downregulated in AD skin samples. Using an air-lift human skin equivalent culture, we demonstrate that NLRP10 promotes keratinocyte survival and is required for epidermal differentiation and barrier function. Mechanistically, NLRP10 limits cell death by preventing the recruitment of caspase-8 to the death inducing signaling complex (DISC) and by inhibiting its subsequent activation. NLRP10 also stabilizes p63, the master regulator of keratinocyte differentiation, to drive proper keratinocyte differentiation and to reinforce the barrier function. Our findings underscore NLRP10 as a key player in atopic dermatitis pathogenesis, highlighting NLRP10 as a potential target for therapeutic intervention to restore skin barrier function and homeostasis in AD.
© 2024. The Author(s).
Conflict of interest statement
All authors are employees of Amgen Inc., a for-profit organization.
Figures


References
-
- Langan SM, Irvine AD, Weidinger S. Atopic dermatitis. Lancet. 2020;396:345–60. - PubMed
-
- Cork MJ, Danby SG, Vasilopoulos Y, Hadgraft J, Lane ME, Moustafa M, et al. Epidermal barrier dysfunction in atopic dermatitis. J Invest Dermatol. 2009;129:1892–908. - PubMed
-
- Boguniewicz M, Fonacier L, Guttman-Yassky E, Ong PY, Silverberg JI. Atopic dermatitis yardstick update. Ann Allergy Asthma Immunol. 2023;130:811–20. - PubMed
-
- Weidinger S, Beck LA, Bieber T, Kabashima K, Irvine AD. Atopic dermatitis. Nat Rev Dis Prim. 2018;4:1. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

