Longitudinal monitoring of circulating tumor DNA to detect relapse early and predict outcome in early breast cancer
- PMID: 39424680
- PMCID: PMC11785695
- DOI: 10.1007/s10549-024-07508-2
Longitudinal monitoring of circulating tumor DNA to detect relapse early and predict outcome in early breast cancer
Abstract
Purpose: Detection of molecular residual disease (MRD) allows for the identification of breast cancer patients at high-risk of recurrence, with the potential that early initiation of treatment at early stages of relapse could improve patient outcomes. The Invitae Personalized Cancer Monitoring™ assay (PCM) is a newly developed next-generation sequencing approach that utilizes up to 50 patient-specific, tumor-informed DNA variants, to detect circulating tumor DNA (ctDNA). The ability of the PCM assay to detect MRD before clinical relapse was evaluated.
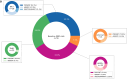
Methods: The cohort included 61 female patients with high-risk breast cancer who underwent neoadjuvant chemotherapy. Plasma samples were collected before and during neoadjuvant therapy, after surgery and during monitoring. PCM was used to detect ctDNA at each time point.
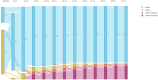
Results: The sensitivity to detect ctDNA in plasma from patients who relapsed during the monitoring phase was 76.9% (10/13). Specificity and positive predictive values were both 100% with all (10/61, 16%) of the patients who had ctDNA detected during the monitoring phase subsequently relapsing. Detection of ctDNA during monitoring was associated with a high-risk of future relapse (HR 37.2, 95% CI 10.5-131.9, p < 0.0001), with a median lead-time from ctDNA detection to clinical relapse of 11.7 months.
Conclusion: PCM detected ctDNA in patients who relapsed with a long lead-time over clinical relapse, shows strong association with relapse-free survival and may be used to identify patients at high-risk for relapse, allowing for earlier intervention.
Keywords: Breast cancer; CtDNA; Liquid biopsy; Minimal residual disease; Relapse.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Competing interests: Robert Daber, Benjamin Hubert, Peter DeFord, David Wooten, Jianhua Zhao, and Rachel E. Ellsworth are current employees of Labcorp Genetics, Inc. Robert Daber, Benjamin Hubert, Chiharu Graybill, Peter DeFord, David Wooten, Jianhua Zhao, Rachel E. Ellsworth and W. Michael Korn are former employees and shareholders of Invitae Corp. Ethical approval: This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Research Ethics Committee ref. no. 11/EE/0063. Consent to participate: Written informed consent was obtained from all participants.
Figures



References
-
- Habbous S, Barisic A, Homenauth E et al (2023) Estimating the incidence of breast cancer recurrence using administrative data. Breast Cancer Res Treat 198:509–522 - PubMed
-
- Gradishar WJ, Moran MS, Abraham J et al (2023) NCCN Guidelines® Insights: Breast Cancer, Version 4.2023. J Natl Compr Canc Netw 21(6):594–608. 10.6004/jnccn.2023.0031 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

