Mapping RANKL- and OPG-expressing cells in bone tissue: the bone surface cells as activators of osteoclastogenesis and promoters of the denosumab rebound effect
- PMID: 39424806
- PMCID: PMC11489716
- DOI: 10.1038/s41413-024-00362-4
Mapping RANKL- and OPG-expressing cells in bone tissue: the bone surface cells as activators of osteoclastogenesis and promoters of the denosumab rebound effect
Abstract
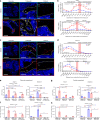
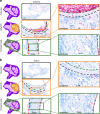
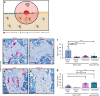
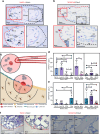
Denosumab is a monoclonal anti-RANKL antibody that inhibits bone resorption, increases bone mass, and reduces fracture risk. Denosumab discontinuation causes an extensive wave of rebound resorption, but the cellular mechanisms remain poorly characterized. We utilized in situ hybridization (ISH) as a direct approach to identify the cells that activate osteoclastogenesis through the RANKL/OPG pathway. ISH was performed across species, skeletal sites, and following recombinant OPG (OPG:Fc) and parathyroid hormone 1-34 (PTH) treatment of mice. OPG:Fc treatment in mice induced an increased expression of RANKL mRNA mainly in trabecular, but not endocortical bone surface cells. Additionally, a decreased expression of OPG mRNA was detected in bone surface cells and osteocytes of both compartments. A similar but more pronounced effect on RANKL and OPG expression was seen one hour after PTH treatment. These findings suggest that bone surface cells and osteocytes conjointly regulate the activation of osteoclastogenesis, and that OPG:Fc treatment induces a local accumulation of osteoclastogenic activation sites, ready to recruit and activate osteoclasts upon treatment discontinuation. Analysis of publicly available single-cell RNA sequencing (scRNAseq) data from murine bone marrow stromal cells revealed that Tnfsf11+ cells expressed high levels of Mmp13, Limch1, and Wif1, confirming their osteoprogenitor status. ISH confirmed co-expression of Mmp13 and Tnfsf11 in bone surface cells of both vehicle- and OPG:Fc-treated mice. Under physiological conditions of human/mouse bone, RANKL is expressed mainly by osteoprogenitors proximate to the osteoclasts, while OPG is expressed mainly by osteocytes and bone-forming osteoblasts.
© 2024. The Author(s).
Conflict of interest statement
M.M.M. and X.G.D. have received honoraria from Amgen. J.F.C. has participated in Alexion, Kyowa Kirin and Amgen advisory boards. J.F.C. has received funding from Novartis. M.B. and J.S.T. have received a research grant from Keros Therapeutics for an unrelated study. T.L.A. has collaborated with Amgen and is the custodian of the biopsies from the FREEDOM study, and in part received free reagents from ACD bioscience (now part of the Bio-Techne Corporation) for the specific study. Amgen Inc provided the OPG:Fc under a material transfer agreement.
Figures





References
-
- Delaisse, J. M. et al. Re-thinking the bone remodeling cycle mechanism and the origin of bone loss. Bone141, 115628 (2020). - PubMed
-
- FROST, H. M. Dynamics of bone remodeling. Bone Biodyn. 315-334 (1964).
-
- Lassen, N. E. et al. Coupling of bone resorption and formation in real time: New knowledge gained from human Haversian BMUs. J. Bone Min. Res.32, 1395–1405 (2017). - PubMed
-
- Parfitt, A. M. The coupling of bone-formation to bone-resorption - a critical analysis of the concept and of its relevance to the pathogenesis of osteoporosis. Metab. Bone Dis. Relat.4, 1–6 (1982). - PubMed
-
- Andersen, T. L. et al. Understanding coupling between bone resorption and formation: Are reversal cells the missing link? Am. J. Pathol.183, 235–246 (2013). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

