T cell immuno-phenotyping : a source of predictive biomarkers for autoimmune hepatitis relapse
- PMID: 39424872
- PMCID: PMC11489469
- DOI: 10.1038/s41598-024-75624-6
T cell immuno-phenotyping : a source of predictive biomarkers for autoimmune hepatitis relapse
Abstract
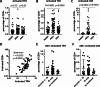
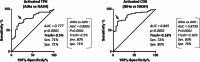
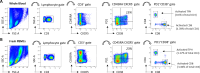
Relapse after immunosuppression (IS) treatment withdrawal is frequent in patients with Autoimmune Hepatitis (AIH), and non-invasive biomarkers predictive of this risk are lacking. We assessed the frequency of circulating T cell subsets as potential biomarkers of disease activity and predictor of the risk of relapse after IS withdrawal. Serum levels of the cytokine B-cell Activating Factor (BAFF) were also investigated. Blood samples from 58 patients with active AIH, 56 AIH patients in remission, and 31 patients with NASH were analyzed. The frequency of activated CD4+ T peripheral helper (TPH) cells (CD4+CD45RA-CXCR5-PD1+CD38+) and of activated CD8+ T cells (CD8+CD45RA-PD1+CD38+) were assessed by flow cytometry. BAFF levels were determined by ELISA. Activated TPH and CD8+ T cell frequencies were significantly increased in patients with active AIH compared to remission AIH or NASH (TPH: 0.88% of total CD3+ vs. 0.42% and 0.39% respectively, p < 0.0001; CD8+ subset: 1.42% vs. 0.09% and 0.11% p < 0.0001). Among patients in remission undergoing treatment withdrawal (n = 18), those with increased frequencies of activated TPH (> 0.5% of total CD3+) and/or activated CD8+ T cells (> 0.18% total CD3+) had a higher risk of relapse (80% vs. 15% after 2 years, p = 0.0071). High BAFF serum concentration (> 213pg/ml) was also associated to a higher risk of relapse (57% vs. 11%, p = 0.0452). In conclusion, high frequency of activated TPH and of activated CD8+, as well as high levels of BAFF, before IS discontinuation, were significantly associated to a greater risk of relapse during the first two years. Thus, they represent promising biomarkers to provide personalized clinical follow-up for patients with AIH.
Keywords: BAFF; Biomarkers; CD38; Peripheral helper T cells; Personalized medicine.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Floreani, A. et al. Etiopathogenesis of autoimmune hepatitis. J. Autoimmun.95, 133–143 (2018). - PubMed
-
- Cardon, A., Conchon, S. & Renand, A. Mechanisms of autoimmune hepatitis. Curr. Opin. Gastroenterol.37, 79–85 (2020). - PubMed
-
- Van Gerven, N. M. F. et al. Relapse is almost universal after withdrawal of immunosuppressive medication in patients with autoimmune hepatitis in remission. J. Hepatol.58, 141–147 (2013). - PubMed
-
- Hegarty, J. E., Nouri Aria, K. T., Portmann, B., Eddleston, A. L. & Williams, R. Relapse following treatment withdrawal in patients with autoimmune chronic active hepatitis. Hepatology3, 685–689 (1983). - PubMed
-
- Hartl, J. et al. Patient selection based on treatment duration and liver biochemistry increases success rates after treatment withdrawal in autoimmune hepatitis. J. Hepatol.62, 642–646 (2015). - PubMed
MeSH terms
Substances
Grants and funding
- ANR-19-CE17-0024-01/Agence Nationale de la Recherche
- ANR-19-CE17-0024-01/Agence Nationale de la Recherche
- ANR-19-CE17-0024-01/Agence Nationale de la Recherche
- ANR-19-CE17-0024-01/Agence Nationale de la Recherche
- ANR-19-CE17-0024-01/Agence Nationale de la Recherche
- ANR-19-CE17-0024-01/Agence Nationale de la Recherche
- ANR-19-CE17-0024-01/Agence Nationale de la Recherche
- ANR-19-CE17-0024-01/Agence Nationale de la Recherche
- ANR-19-CE17-0024-01/Agence Nationale de la Recherche
- ANR-19-CE17-0024-01/Agence Nationale de la Recherche
- ANR-19-CE17-0024-01/Agence Nationale de la Recherche
- ANR-19-CE17-0024-01/Agence Nationale de la Recherche
- ANR-19-CE17-0024-01/Agence Nationale de la Recherche
- ANR-19-CE17-0024-01/Agence Nationale de la Recherche
- ANR-19-CE17-0024-01/Agence Nationale de la Recherche
- ANR-19-CE17-0024-01/Agence Nationale de la Recherche
- ANR-19-CE17-0024-01/Agence Nationale de la Recherche
LinkOut - more resources
Full Text Sources
Research Materials

