Trophoblast cell-derived extracellular vesicles regulate the polarization of decidual macrophages by carrying miR-141-3p in the pathogenesis of preeclampsia
- PMID: 39424901
- PMCID: PMC11489854
- DOI: 10.1038/s41598-024-76563-y
Trophoblast cell-derived extracellular vesicles regulate the polarization of decidual macrophages by carrying miR-141-3p in the pathogenesis of preeclampsia
Abstract
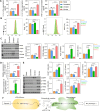
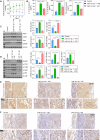
Dysregulation of macrophage polarization can prevent the invasion of trophoblast cells and further limit spiral artery remodeling in preeclampsia (PE). However, its mechanism is obscure. HTR8-/Svneo cells were cultured under normoxic or hypoxic conditions and extracellular vesicles (EVs) in the culture supernatants were extracted. Next, the cells were incubated with those EVs to investigate their effects on trophoblasts. A co-culture system consisting of HTR8-/Svneo cells and macrophages was used to reveal how the trophoblast-derived EVs affected the macrophage subtype. Finally, a PE mouse model and miR-141-3p knockout mice were used to verify the function of miR-141-3p in PE. Hypoxia induced abnormal increases in the levels of miR-141-3p in HTR8-/Svneo cells and EVs. EVs from hypoxia-treated HTR8-/Svneo cells could downregulate PTEN, a potential target of miR-141-3p, and inhibit trophoblast mitophagy and invasion. However, HTR8-/Svneo cells transfected with an miR-141-3p inhibitor could attenuate the influence of EVs. In an HTR8-/Svneo cell plus macrophage co-culture system, hypoxia-pretreated cells promoted the transformation of macrophages into the M1-phenotye, and HTR8-/Svneo invasion was inhibited by the macrophages. MiR-141 from EVs could target and downregulate dual specificity phosphatase 1 (DUSP1) expression in macrophages, induce formation of the M1 macrophage phenotype in THP-1 cells, downregulate DUSP1 expression, and upregulate TAB2/TAK1 signaling. These results were also demonstrated in normal pregnant mice and PE pregnant mice. A hypoxic environment could upregulate miR-141 expression in the EVs of HTR8-/Svneo cells, and THP-1-derived macrophages could uptake EVs releasing miR-141 to downregulate DUSP1 expression and induce the formation of M1 macrophages, which can lead to the development of PE.
Keywords: Angiogenesis; Exosome; Macrophage; Preeclampsia; Trophoblast.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures







References
-
- Chappell, L. C., Cluver, C. A., Kingdom, J. & Tong, S. Pre-eclampsia. Lancet. 398 (10297), 341–354 (2021). - PubMed
-
- Pineles, B. L. et al. Distinct subsets of microRNAs are expressed differentially in the human placentas of patients with preeclampsia. Am. J. Obstet. Gynecol. 196(3), 261e261–261e266 (2007). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

