Glycogenesis and glyconeogenesis from glutamine, lactate and glycerol support human macrophage functions
- PMID: 39424955
- PMCID: PMC11624281
- DOI: 10.1038/s44319-024-00278-4
Glycogenesis and glyconeogenesis from glutamine, lactate and glycerol support human macrophage functions
Abstract
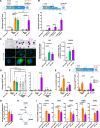
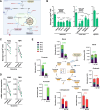
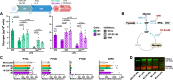
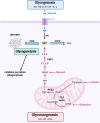
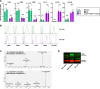
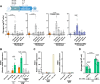
Macrophages fight infection and ensure tissue repair, often operating at nutrient-poor wound sites. We investigated the ability of human macrophages to metabolize glycogen. We observed that the cytokines GM-CSF and M-CSF plus IL-4 induced glycogenesis and the accumulation of glycogen by monocyte-derived macrophages. Glyconeogenesis occurs in cells cultured in the presence of the inflammatory cytokines GM-CSF and IFNγ (M1 cells), via phosphoenolpyruvate carboxykinase 2 (PCK2) and fructose-1,6-bisphosphatase 1 (FBP1). Enzyme inhibition with drugs or gene silencing techniques and 13C-tracing demonstrate that glutamine (metabolized by the TCA cycle), lactic acid, and glycerol were substrates of glyconeogenesis only in M1 cells. Tumor-associated macrophages (TAMs) also store glycogen and can perform glyconeogenesis. Finally, macrophage glycogenolysis and the pentose phosphate pathway (PPP) support cytokine secretion and phagocytosis regardless of the availability of extracellular glucose. Thus, glycogen metabolism supports the functions of human M1 and M2 cells, with inflammatory M1 cells displaying a possible dependence on glyconeogenesis.
Keywords: Cytokine secretion; Glycogenolysis; Glyconeogenesis; Macrophages; Phagocytosis.
© 2024. The Author(s).
Conflict of interest statement
Disclosure and competing interests statement. The authors declare no competing interests.
Figures






References
-
- Belal S, Goudenège D, Bocca C, Dumont F, Chao De La Barca JM, Desquiret-Dumas V, Gueguen N, Geffroy G, Benyahia R, Kane S et al (2022) Glutamate-induced deregulation of Krebs cycle in mitochondrial encephalopathy lactic acidosis syndrome stroke-like episodes (MELAS) syndrome is alleviated by ketone body exposure. Biomedicines 10:1665 - PMC - PubMed
-
- Bultot L, Guigas B, Von Wilamowitz-Moellendorff A, Maisin L, Vertommen D, Hussain N, Beullens M, Guinovart JJ, Foretz M, Viollet B et al (2012) AMP-activated protein kinase phosphorylates and inactivates liver glycogen synthase. Biochem J 443:193–203 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

