Comparative evaluation of autologous tissue-engineered ocular and oral mucosal tissue grafts- a prospective randomized controlled trial
- PMID: 39425087
- PMCID: PMC11488145
- DOI: 10.1186/s12896-024-00876-z
Comparative evaluation of autologous tissue-engineered ocular and oral mucosal tissue grafts- a prospective randomized controlled trial
Abstract
Background: Bilateral ocular surface disease resulting from Stevens Johnson Syndrome (SJS) and chemical injuries are visually debilitating and difficult to treat. Ocular surface reconstruction by various means has been reported with variable results. This study addresses an unmet need for a prospective clinical trial comparing the outcomes of transplanting autologous oral and conjunctival epithelial cell constructs on human amniotic membrane by ex vivo tissue engineering.
Methods: A prospective, randomized controlled clinical trial was prospectively applied for registration, with the clinical trial registry of India (CTRI), with the approval of the Institute Ethics Committee number IEC/NP-99/11.04.2014 and CTRI No. REF/2018/10/021791, the study also registered with the WHO-recognized trial registry, International Standard Randomised Controlled Trial Number (ISRCTN) registration reference number 45780. The study was conducted to compare clinical outcomes of two different tissue-engineered cell grafts, Cultivated Oral Mucosal Epithelial Transplantation (COMET) and Conjunctival Cultivated Epithelial Transplantation (CCET) for ocular surface reconstruction in patients with bilateral ocular surface disease due to Stevens-Johnson Syndrome or chemical injuries. Fifty patients were enrolled and randomized to either the COMET or CCET group. A uniform pre-op and post-op protocol using standard medications was followed for all patients Parameters assessed at baseline, day 1, 1 week, 2 weeks, 1 month, 2 months, 3 months and 6 months postoperatively included patient comfort, best corrected visual acuity (BCVA), ocular surface status and corneal clarity. The efficacy was measured in terms of improvement of vision, reduction in vascularization, symblepharon and corneal clarity.
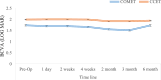
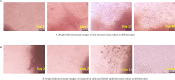
Results: In the study, 50 patients (50 eyes; mean ages of 29 ± 15.86 years and 26.36 ± 10.85 years, respectively; range, 12-65 years) were enrolled, with 25 patients each in the COMET and CCET groups. Out of them, 36% were female and 64% were male; the causes were Steven Johnson syndrome (48), and chemical injury (2). Mean pre-operative BCVA was log MAR 1.73 ± 0.57 for COMET and 1.99 ± 0.33 for the CCET group. Pre-operatively all 50 enrolled patients had opaque corneas pre-operatively, symblepharon that extended to the cornea categorised as grade 3 and corneal vascularization that went beyond the pupil's boundary into the central zone encluaching on the visual axis. The minimal follow-up time was six months. Following surgery postoperatively, the BCVA considerably improved in the COMET group by 1.51 ± 0.58 compared to the CCET group by 1.91 ± 0.33 at 3 months. BCVA at 6 months was 1.73 ± 0.56 in the COMET group and 1.99 ± 0.31 in the CCET group, which is not statistically significant and comparable to the BCVA before surgery. The corneal clarity was significantly improved in COMET group 25 eye (100%) at 2 month, 3month and 19 eye (76%), 6eye (24%) at 6 months when compared to CCET group 15 eye improved (60%), 9 eyes (36%) not improved and one eye with opaque cornea (4%) at 2 months. 22 eye (88%) had not improved, 2 eye (8%) opaque cornea and 1 eye (4%) improved at 3 months. At 6 months 21 eye (84%) were not improved, 4 eye (16%) eye became opaqued at 6 months. Compared to preoperative conditions, both groups had improved corneal clarity significantly (p > 0.005). Of the 50 patients with grade 3 symblepharon extended to the cornea, were completely resolved 19 (76%) in COMET group when compared to CCET group 22 eye (88%) not improved. Similarly, 19 eye (76%) had a improvement in corneal vascularization when compared to the CCET group not improved 25 eye (100%) at 6months. No adverse event was observed in any of either group during the follow up periods.
Conclusion: Both cell types are effective to restore the ocular surface integrity in bilateral ocular surface disease. Whereas COMET is safe and efficacious in terms of improvement of clinical parameters including, BCVA, corneal clarity, reduction in vascularization and preventing the recurrence of symblepharon postoperatively 3months and 6 months. In addition, the CCET group maintained the stability of the ocular surface and had improvement in corneal clarity and a decrease in vascularization at 3 months compared to their pre-operative characteristics.
Keywords: Chemical injury; Conjunctival cultivated epithelial transplantation (CCET); Conjunctival epithelial cells; Cultivated oral mucosal epithelial transplantation (COMET); Ocular surface disease (OSD); Oral mucosal epithelial cells; Stevens-johnson syndrome (SJS); Tissue engineering, ex vivo expansion.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Visual improvement after cultivated oral mucosal epithelial transplantation.Ophthalmology. 2013 Jan;120(1):193-200. doi: 10.1016/j.ophtha.2012.07.053. Epub 2012 Oct 16. Ophthalmology. 2013. PMID: 23084239
-
Limbal stem cell transplantation: an evidence-based analysis.Ont Health Technol Assess Ser. 2008;8(7):1-58. Epub 2008 Oct 1. Ont Health Technol Assess Ser. 2008. PMID: 23074512 Free PMC article.
-
Long-term result of autologous cultivated oral mucosal epithelial transplantation for severe ocular surface disease.Cell Tissue Bank. 2016 Sep;17(3):491-503. doi: 10.1007/s10561-016-9575-4. Epub 2016 Aug 9. Cell Tissue Bank. 2016. PMID: 27507558 Clinical Trial.
-
[Ocular surface reconstruction by tissue engineering].Nippon Ganka Gakkai Zasshi. 2002 Dec;106(12):837-68; discussion 869. Nippon Ganka Gakkai Zasshi. 2002. PMID: 12610839 Review. Japanese.
-
[Research and development for treating devastating corneal diseases].Nippon Ganka Gakkai Zasshi. 2010 Mar;114(3):161-99; discussion 200-1. Nippon Ganka Gakkai Zasshi. 2010. PMID: 20387535 Review. Japanese.
References
-
- Dua HS, Miri A, Alomar T, et al. The role of limbal stem cells in corneal epithelial maintenance: testing the dogma. Ophthalmology. 2009;116:856–63. - PubMed
-
- Vazirani J, Nair D, Shanbhag S, et al. Limbal Stem Cell Deficiency-Demography and underlying causes. Am J Ophthalmol. 2018;188:99–103. - PubMed
-
- Choi SH, Kim MK, Oh JY. Corneal Limbal Stem Cell Deficiency in Children with Stevens-Johnson syndrome. Am J Ophthalmol. 2019;199:1–8. - PubMed
-
- Vazirani J, Mariappan I, Ramamurthy S, et al. Surgical Management of bilateral Limbal Stem Cell Deficiency. Ocul Surf. 2016;14:350–64. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

