Intestinal homeostasis disrupted by Periodontitis exacerbates Alzheimer's Disease in APP/PS1 mice
- PMID: 39425119
- PMCID: PMC11489998
- DOI: 10.1186/s12974-024-03256-8
Intestinal homeostasis disrupted by Periodontitis exacerbates Alzheimer's Disease in APP/PS1 mice
Abstract
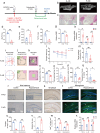
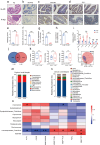
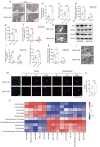
Periodontitis exacerbates Alzheimer's disease (AD) through multiple pathways. Both periodontitis and AD are intricately correlated to intestinal homeostasis, yet there is still a lack of direct evidence regarding whether periodontitis can regulate the progression of AD by modulating intestinal homeostasis. The current study induced experimental periodontitis in AD mice by bilaterally ligating the maxillary second molars with silk and administering Pg-LPS injections in APPswe/PS1ΔE9 (APP/PS1) mice. Behavioral tests and histological analyses of brain tissue were conducted after 8 weeks. Gut microbiota was analyzed and colon tissue were also evaluated. Then, fecal microbiota from mice with periodontitis was transplanted into antibiotic-treated mice to confirm the effects of periodontitis on AD and the potential mechanism was explored. The results indicated periodontitis exacerbated cognitive impairment and anxious behaviour in APP/PS1 mice, with increased Aβ deposition, microglial overactivation and neuroinflammation in brain. Moreover, the intestinal homeostasis of AD mice was altered by periodontitis, including affecting gut microbiota composition, causing colon inflammation and destroyed intestinal epithelial barrier. Furthermore, AD mice that underwent fecal transplantation from mice with periodontitis exhibited worsened AD progression and disrupted intestinal homeostasis. It also impaired intestinal barrier function, elevated peripheral inflammation, damaged blood-brain barrier (BBB) and caused neuroinflammation and synapses impairment. Taken together, the current study demonstrated that periodontitis could disrupt intestinal homeostasis to exacerbate AD progression potential via causing gut microbial dysbiosis, intestinal inflammation and intestinal barrier impairment to induce peripheral inflammation and damage BBB, ultimately leading to neuroinflammation and synapse impairment. It underscores the importance of maintaining both periodontal health and intestinal homeostasis to reduce the risk of AD.
Keywords: Alzheimer’s disease; Gut microbiota; Inflammation; Intestinal homeostasis; Periodontitis.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
The authors assert that they possess no conflicts of interest.
Figures



References
MeSH terms
Substances
Grants and funding
- 81860197/the National Natural Science Foundation of China
- 2020Y9032/the Joint Funds for the Innovation of Science and Technology
- JAT220078/the Educational Research Project for Young and Middle-aged Teachers of Fujian Provincial Department of Education
- 82301103/National Outstanding Youth Science Fund Project of National Natural Science Foundation of China
LinkOut - more resources
Full Text Sources
Medical

